- HYGEIA
- Vision & Mission
- Timeline
- Organizational structure
- Press Releases
- Social responsibility
- Awards and Distinctions
- Human Resources
- Scientific & Training activities
- Articles – Publications
- Our Facilities
- Magazines
- Healthcare Programs
- Doctors
- Services
- Medical Divisions & Services
- Imaging Divisions
- Departments
- Units
- Centers of Excellence
- Emergency – Outpatient
- Nursing Service
- Ambulances
- Patients
- Hygeia
- Υπηρεσίες
- Ιατρικά Τμήματα & Υπηρεσίες
Structural Heart Disease and Transcatheter Heart Valves
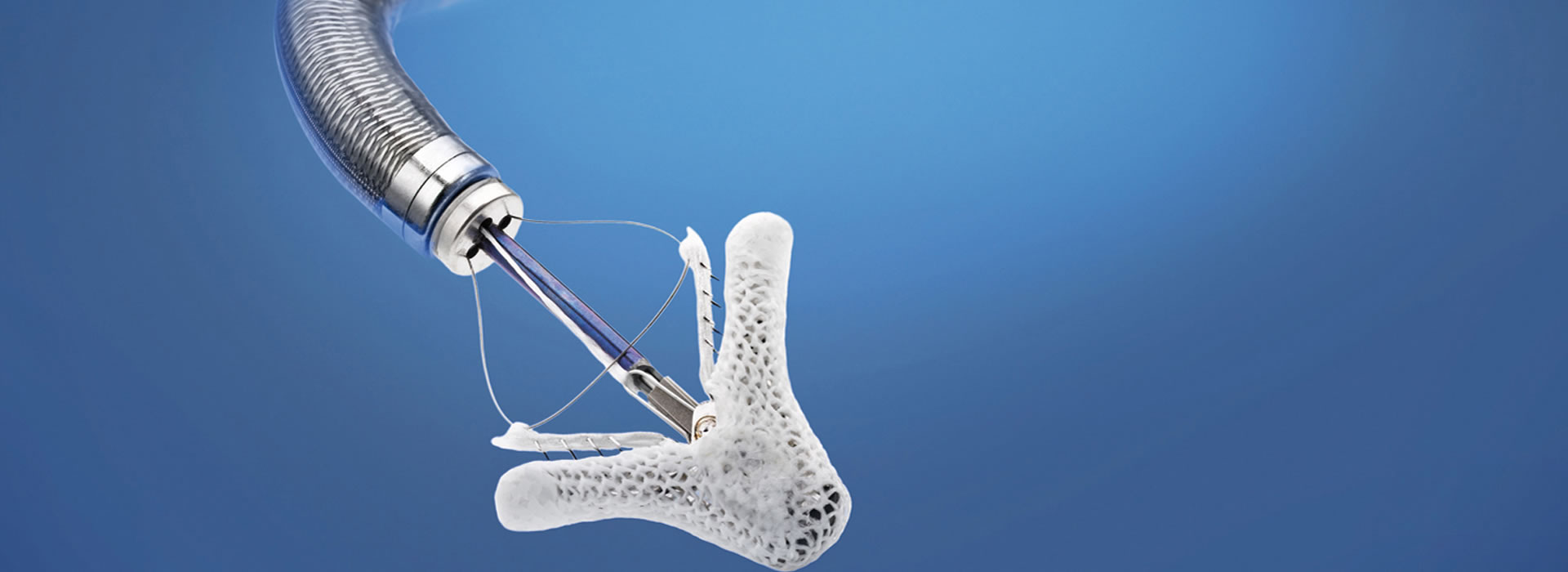
The Transcatheter Heart Valve Department has been operating since 2010. The Department staff have performed more than 2.000 transcatheter aortic valve replacements (the most in Greece), with all types of valves available today (SAPIEN Ultra, Edwards Lifesciences; Evolut PRO, Medtronic; Portico, Abbott; ACURATE neo2, Boston Scientific; JenaValve) and using all possible implantation methods (transfemoral, transaortic, transthoracic or transapical and subclavian route).
The HYGEIA Hospital Transcatheter Heart Valve Department is or has been an official global training center for the SAPIEN 3 valves by Edwards Lifesciences and the Evolut R valves by Medtronic, while its medical staff are worldwide trainers for the placement of valves manufactured by Edwards and Medtronic.
The Transcatheter Heart Valve Department successfully introduced the transcatheter mitral valve regurgitation repair using the MitraClip system (Abbott Vascular) in Greece in 2011, and more recently (2019) the PASCAL system (Edwards Lifesciences), and more than 350 clips have been implanted to this day. The Department medical staff are trainers for the MitraClip and PASCAL procedures.
In addition, the Department performed the first transcatheter tricuspid valve repairs in Greece in 2015, while the program is continuing with the use of various devices (TriClip, PASCAL).
All transcatheter procedures are performed in the state-of-the-art HYGEIA Hybrid Operating Room.
Aortic Valve Stenosis
The aortic valve is one of the 4 valves of the heart and it ensures blood flow from the heart to the rest of the body. The normal aortic valve opening is 3-4 cm2. The stenosis of the valve is considered significant when the opening is reduced to less than 1 cm2.
Image 1. Progressive Aortic valve stenosis
The degenerative mechanism that causes aortic valve stenosis (narrowing) is dynamic and evolving. The main features of this process are gradual stiffening and thickening of the valve with calcium deposits that progressively limit leaflet mobility and eventually narrow the valve. Approximately 5% of the elderly population (>75 years) have developed at least moderate aortic valve stenosis.
When the condition becomes severe, it develops rapidly and the prognosis is poor, with high morbidity and hassles for the patients and their families.
The most common symptoms of aortic valve stenosis are shortness of breath, angina and dizziness, up to syncopal attacks. After the onset of symptoms, average patient survival drops drastically.
Treatment
Pharmaceutical treatment can relieve some of the symptoms temporarily, but it does not affect or treat the valve condition. The only treatment is valve replacement, which could only be performed with open surgery until a few years ago but more recently can be accomplished with catheters percutaneously.
Many patients are already of an advanced age when the symptoms appear and often suffer from other conditions that greatly increase the risk of conventional cardiac surgery, or even prohibit it. Therefore, surgical replacement is considered too risky in these patients and they often do not undergo surgery (it is estimated that 1 in every 2 patients who must undergo conventional surgical replacement of aortic valve is rejected). Prognosis in these patients is poor and morbidity long and painful.
Transcatheter Aortic Valve Replacement (Implantation): TAVR or TAVI is being successfully performed since 2007, initially on patients who could not have been treated in the past. Since 2011, TAVI has also been approved and is successfully performed on patients who could have undergone surgical replacement, but were considered high risk. With low single-digit mortality rates and fast recovery and return to daily activities, the benefits of this method are obvious.
In 2016, the results of the first clinical trials showed better survival with TAVR compared to the surgical replacement, even in lower (moderate) risk patients, and the use of the method has since been extended to these patients as well. In 2018, newer clinical trials produced similar results for the TAVI method in low-risk patients (essentially for all patients now). As a result, the method was approved and indicated by the FDA in 2019 for application on essentially all patients with aortic valve stenosis.
Most recent advances and study data led to the 2021 Treatment Guidelines that grant the strongest level of indication to TAVI in patients older than 65 years (in USA, ACC/AHA) or 75 years (in Europe, ESC)
Image 2. Images of the most often implanted transcatheter aortic valves (SAPIEN Ultra, Evolut PRO, ACURATE neo2, Navitor)
The Studies
The Transcatheter Aortic Valve Replacement is the most researched valve replacement method, as well as one of the most researched treatments in Cardiology and Cardiac Surgery in general.
The results of the randomized PARTNER-B study were announced in 2010, when it was found that the transcatheter method for treating aortic valve stenosis is changing the natural history of the disease and improves survival. Older patients with coexisting conditions, who would generally be treated conservatively due to the forbiddingly high surgical risk, underwent randomized transfemoral valve implantation and showed remarkable improvement of symptoms, reduced need for hospitalization by 54% and reduced morbidity by 45% as early as the first year. Consequently, the method was recognized for this patient population, who would otherwise be doomed to severe morbidity and very poor prognosis. It is estimated that there are at least 1,000 such patients in Greece (per year) who could benefit from the transcatheter approach.
The results of the randomized PARTNER-A study were announced in March 2011. PARTNER-A is significant in that it randomized high-risk patients (Logistic Euroscore ≥ 15%), who, however, were not rejected for surgical treatment. 30-day morbidity for the transcatheter procedure (3.4%) was almost 50% lower compared to the corresponding surgical replacement (6.7%), proving that they are at least equivalent treatments. Survival in the first year was 2.6% higher for the transcatheter method. Especially with regard to the transfemoral implantation, 30-day survival was significantly higher compared to the surgical replacement.
The results of PARTNER-A provided the first comparative assessment of the transcatheter implantation compared to the surgical replacement of the aortic valve, demonstrating that even high-risk patients who can undergo surgery enjoy just as good results with transcatheter implantation, and with all the benefits of a less invasive method. This population is estimated at around 25% of patients who undergo surgical replacement to date (patients with a Logistic Euroscore ≥ 15%) (approximately 300 patients/year in Greece, on top of the ~1000 deemed inoperable).
The first randomized clinical study that proved the superiority of the transcatheter method over the conventional surgical replacement was announced in the spring of 2014 (CoreValve US Pivotal High Risk Study). In this study, 795 high-risk patients (Logistic Euroscore average of 18%) who were eligible for conventional surgery were randomized to transcatheter or surgical replacement. The morbidity of those who underwent the transfemoral method was significantly lower both at 1 month (3.3% for the transcatheter, 4.5% for the surgical) and at 1 year (14.2% for the transcatheter, 19.1% for the surgical).
The results of the latest generation SAPIEN 3 valve by Edwards Lifesciences on 1,655 high and medium surgical risk patients were announced in the beginning of 2016. This study showed the lowest 30-day morbidity rates to date, i.e. 1.6% for the transfemoral procedure in high-risk patients and 1.1% in medium-risk patients.
The results of the PARTNER 2 study, which randomized 2,032 medium-risk patients (STS score 4%-8%) to transcatheter or surgical aortic valve replacement, were also announced in the beginning of 2016. The patients who underwent transfemoral valve implantation had better early survival as opposed to those who underwent conventional surgical replacement. The results of these studies provided the indication and established the transcatheter replacement in medium-risk patients (around 25% of patients undergoing surgical replacement up until that time).
The results of the PARTNER 3 and the EVOLUT Low Risk Trials were both announced at the beginning of 2019. They included 2,468 low-risk patients (STS < 4%) and during the 2-year follow-up, they showed that not only is TAVR safe and effective in these patients as well, but the incidence of death or stroke was halved compared to the conventional surgical replacement (PARTNER 3 and EVOLUT Low Risk Trials combined: 2.6% vs. 5.2%). In practical terms this means that if 200 such patients undergo TAVR as opposed to conventional surgery, 5 more will return for the 2-year follow-up alive and without having suffered a stroke. These concrete results lead to a more generalized application of the TAVR in patients with aortic valve stenosis.
As of 2007, there has been wide clinical acceptance of the method (initially in Europe, followed by the USA and Japan) and the number of procedures performed followed a geometric progression, with an 40%-80% rise annually. More than 1.500.000 implantations have been performed to date (10/2021) globally (4.000 in Greece). In many countries, transcatheter implantations have clearly exceeded the surgical ones. In most western European countries, 200-400 transcatheter implantations are performed per 1 million population annually.
With regard to the clinical results in everyday practice, the announcements from the most recent register recordings and the studies show that 30-day morbidity in high/medium-risk patients who undergo transfemoral transcatheter replacement is around 2%-3%, while it is 1% in low-risk patients. The recorded 30-day morbidities for transthoracic and transaortic implantations are 3%-6%, subject to the baseline patient risk.
Transcatheter implantations of all valve types currently available are performed at HYGEIA Hospital (Image 2), using all possible implantation methods (transfemoral, transaortic, transthoracic, subclavian route etc.). The Department staff’s experience exceeds 2.000 procedures, while 30-day morbidity of transfemoral procedures has been < 2%.
Transcatheter Heart Valve Durability
There is plenty of detailed follow-up information on many patients who carry transcatheter valves in the last 10 years and there have been no concerns as to their long-term resilience and functionality. Besides, they are manufactured by the same companies that manufacture surgical valves and undergo the same rigorous bench testing. It is expected that they will continue to operate well for just as long (i.e. 10-16 years). However, even if they malfunction, this will happen gradually and it will be possible to replace them with new transcatheter valves that will be implanted in the same simple manner. This is already being done on patients with malfunction of older surgically placed bioprosthetic valves, however, this time these patients are treated with the transcatheter method rather than undergoing a new surgical procedure.
Aortic Valve Regurgitation
Although it is rarer than aortic valve stenosis, aortic valve regurgitation causes the same serious problems and treatment is once again replacement of the faulty valve. Provided it can be performed, transcatheter replacement is the best choice for higher-risk patients.
The Procedure
Transfemoral procedure
The procedure is performed through the femoral artery, similarly to a coronary angiography. Strict antisepsis measures are in place in the HYGEIA Hospital Hybrid Operating Room, where the procedure is performed. General anesthesia and intubation are not required, but patients are placed under mild sedation, so that they remain calm and can cooperate. The procedure usually lasts less than 30 minutes, but patients remain in the operating room for about 90 minutes total (preparation upon arrival and departure). The entry point of the valve into the femoral artery is sealed externally, while there is no incision on the skin.
Patients remain on bed rest for the first night postoperatively, at the Intensive Care Unit or High Dependency Unit, and then they are fully mobile and remain hospitalized in a simple ward for another 1-3 days. In the vast majority of patients (> 97%), the femoral arteries are suitable for valve implantation. It is generally accepted that transfemoral implantations are preferable, as they are less invasive and carry a lower risk.
Transaxillary, Transaortic and Transapical (transthoracic) procedure
Following the advancement and improvement of transcatheter valves, transaxillary, transaortic and transapical implantations are rarely performed these days (less than 5% of patients), and only when the femoral arteries are deemed unsuitable for transfemoral implantation (due to small size, stenosis, etc.). The procedure is performed under general anesthesia and the valve is implanted either through the axillary artery (transaxillary) or the aorta (transaortic), through a small incision between the ribs at the top of the chest, or directly in the heart (transapical), through a small incision between the ribs on the left, under the nipple. The procedure usually lasts less than 1 hour, but patients remain in the operating room for about 2 hours total (preparation upon arrival and departure). The incision on the skin between the ribs is around 3-5 cm long and is sutured closed. Patients remain on bed rest in the Intensive Care Unit for the first day postoperatively and then they are hospitalized in a simple ward for another 2-3 days.
These patients also enjoy the key benefits of the method, avoiding a sternotomy and extracorporeal circulation.
Eligibility testing
A patient’s eligibility is assessed through a series of tests as an outpatient, and rarely is 1-day hospitalization required. The tests include coronary angiography to check the heart arteries (invasive or bloodless with CT), cardiac CT, CT angiography of the limbs to determine their suitability for valve insertion, echocardiography, blood tests and any other exams deemed necessary, depending on each patient. Patients are also evaluated by the entire medical team that will participate in the procedure (cardiologists, cardiac surgeons, anesthesiologists, vascular surgeons, etc., i.e. the Heart Team). After testing, they decide on: the implantation method (transfemoral, transaortic, transapical, etc.), most suitable valve type (for anatomical and other reasons, one type of valve is often preferable to another in each patient) and suitable valve size.
Through this procedure, it is also possible to manage any significant stenoses encountered in the coronary arteries using angioplasty and stents. Finally, palliative valvuloplasty (valve opening with balloon) may be performed on gravely ill patients, so they may undergo valve implantation at a later date.
Mitral Valve Regurgitation
It is the most frequent valve disease and it is estimated that 8%-10% of people aged over 75 may develop at least moderate mitral valve regurgitation. The inability of this valve to close tightly results in part of the blood, which was to be channeled to the entire body through the aorta, flowing back into the lungs.
Image 1. Illustration of Mitral valve regurgitation (insufficiency)
Severe mitral valve regurgitation causes heart failure symptoms (fatigue and shortness of breath during natural fatigue, up to acute pulmonary edema) and predisposition for developing atrial fibrillation (arrhythmia) and stroke.
Mitral valve regurgitation is distinguished into functional (most prevalent, usually due to coronary disease or dilated cardiomyopathy) and organic (often degenerative, which causes leaflet prolapse). Pharmaceutical treatment may manage and temporarily improve some of the symptoms caused by severe mitral valve regurgitation. However, given that the problem is primarily mechanical, it cannot treat it.
Surgical treatment of the functional regurgitation of the mitral valve is attempted only when the patient also suffers from coronary artery disease and must undergo aortocoronary bypass surgery for precisely this reason. Otherwise the benefit is unconfirmed and the risk of the procedure high. This is also the reason why patients rarely undergo surgical procedure just to treat the functional valve regurgitation. Transcatheter repair is most applicable to them.
Surgical treatment of organic valve regurgitation usually involves open or endoscopic surgical repair. However, at times, this is not possible, in which case, it is replaced by a prosthetic valve. Nevertheless, there are many such patients who do not eventually undergo surgical treatment because they are deemed high risk for various reasons. Transcatheter repair is also applicable to them.
The percutaneous repair method used today is called Transcatheter Edge-to-Edge Repair (TEER) and is accomplished with the use of two available devices: MitraClip and PASCAL.
MitraClip: Transcatheter mitral valve regurgitation repair system
The MitraClip system (Abbott Vascular) is based on the transcatheter placement of one or more clips in the mitral valve and aims to reduce or even eliminate the regurgitation. This minimally invasive mitral valve repair mimics the open surgery method by creating a double orifice (Alfieri method) and increases the treatment choices available to patients who suffer from severe valve regurgitation.
Image 2. MitraClip and its delivery catheter
The MitraClip device places a clip in the mitral valve, without opening the patient’s chest and without incisions. The guide catheter is introduced through the femoral vein to reach the heart, similarly to a coronary angiography. The procedure is performed under general anesthesia, without using a heart-lung machine (extracorporeal circulation) and without a sternotomy. Hospitalization is two to three days postoperatively.
It has been proven to reduce the symptoms of heart failure, improve the quality of life and prolongs survival in select patients with functional mitral valve regurgitation. The method is very effective and has extremely low risk for complications.
The MitraClip system consists of three main subsystems (Image 2): A steerable guide catheter. A clip delivery system. A MitraClip device (implant).
The MitraClip is the first transcatheter mitral valve regurgitation repair system to receive the European CE certification in 2008 and be approved by the FDA in 2013, while more than 150,000 procedures have been performed globally to date.
The COAPT clinical trial, reported in 2018, included 603 patients with functional mitral valve regurgitation and compared the MitraClip with the conservative conventional treatment. It is extremely important that treatment using the MitraClip method showed significant and major reduction in morbidity, resulting in the US FDA extending the treatment indications in 2019.
In Greece, these procedures were performed for the first time by the HYGEIA Hospital Transcatheter Heart Valve Department in October 2011 and were met with great success. The program has been successfully continuing since then and more than 350 clips have been implanted.
PASCAL: Transcatheter mitral valve regurgitation repair system
The clinical application of the transcatheter mitral valve regurgitation repair using the PASCAL system by Edwards Lifesciences was approved in February 2019. This system achieves the Edge-to-Edge repair (TEER) similarly to MitraClip, but it has certain features that make more patients amenable to such therapy.
The HYGEIA Hospital Transcatheter Heart Valve Department was among the main investigators of the CLASP trial, which led to the PASCAL system receiving the CE mark due to its excellent results. The PASCAL system is currently used for the transcatheter repair of the mitral valve. The technique is similar to the one used for the MitraClip system, which is being successfully applied at HYGEIA for many years. The first commercial application in Greece was at the HYGEIA Hospital Transcatheter Heart Valve Department in 2019.
Image 3. PASCAL and its delivery catheter
The PASCAL system consists of three main subsystems (Image 3): A deflectable guide catheter. A deflectable delivery system. A PASCAL device (implant).
Possible treatment indications for MitraClip/PASCAL
The implantation of the clip may significantly reduce or even eliminate mitral valve regurgitation in the following cases:
-Significant functional mitral valve regurgitation that does not require surgical treatment of coexisting coronary artery disease.
-Significant organic mitral valve regurgitation in high surgical risk patients.
The anatomical eligibility criteria are examined using transthoracic and intraesophageal echocardiography (Doppler ultrasound).
Tricuspid Valve Regurgitation
It is the second most frequent valve disease following mitral regurgitation. The inability of this valve to close tightly results in part of the blood, which was to be channeled to the lungs, flowing back into the body where it is coming from, resulting in swelling of the legs and the abdomen and congestion to organs such as liver and kidneys.
Image 1. Illustration of Tricuspid valve regurgitation (insufficiency)
Severe tricuspid valve regurgitation causes heart failure symptoms such as fluid accumulation (swelling) in the legs and abdomen (ascites), fatigue and shortness of breath and muscular weakness. The symptoms can be mild and insidious despite the underlying severity of the valve disease. With time the right heart becomes overloaded with the excessive blood volumes it handles, dilates and weakens. The symptoms and signs then become pronounced and organ failure such as liver and renal failure may develop. If treatment comes too late the situation may already be irreversible.
Tricuspid valve regurgitation is usually secondary to other diseases that impact on the tricuspid valve. Usual culprits are left heart failure, coronary artery disease, aortic or mitral valve disease, pulmonary hypertension and chronic atrial fibrillation. Drug therapy is usually diuretics to remove the excess body fluid and temporarily relieve symptoms.
Surgical treatment of the tricuspid valve is not attempted that often and usually only in combination with other necessary heart surgery such as for other valve disease or for coronary bypass. Part of the reluctance to refer these patients for surgery is the long-term cumulative data showing that tricuspid valve surgery is high risk and associated with a 6-10% mortality (irrespective of whether it is open chest or endoscopic, and repair or replacement). This and the long lasting and insidious initial stage of the disease are the reasons that patients and doctors alike rarely consider surgical therapy for the tricuspid valve, which is often referred as the forgotten valve.
For all the above-mentioned reasons an effective tricuspid regurgitation transcatheter therapy with low risk and fast recovery is highly desirable and potentially applicable to most patients.
After many years of research and experimentation with various devices and methods the transcatheter technique that prevailed today is the edge-to-edge leaflet repair (TEER). This can be accomplished with two approved (since 2020) clip devices: the TriClip and the PASCAL. These therapies are available and employed only in highly specialized centers with extensive experience. It is performed in our department since 2018.
The transcatheter therapy of tricuspid regurgitations is included for the first time in the official Treatment Guidelines of the European Society of Cardiology (2021)
TEER for the treatment of tricuspid valve regurgitation has been shown to alleviate fluid retention, swelling and heart failure symptoms and greatly improve the life quality of these patients. Overall, TEER is an effective and benign low risk therapy.
TriClip: Transcatheter tricuspid valve regurgitation repair system
The TriClip system (Abbott Vascular) is based on the transcatheter placement of one or more clips in the tricuspid valve and aims to reduce or even eliminate the regurgitation. The guide catheter is introduced through the femoral vein to reach the heart, similarly to a coronary angiography. The procedure is performed under general anesthesia, without using a heart-lung machine (extracorporeal circulation) and without a sternotomy. Hospitalization is two to three days postoperatively.
Image 2. TriClip and its delivery catheter
The TriClip system consists of three main subsystems (Image 2): A steerable guide catheter. A clip delivery system. A TriClip device (implant).
PASCAL: Transcatheter tricuspid valve regurgitation repair system
The PASCAL system by Edwards Lifesciences was also approved for tricuspid use in 2020.This system achieves similar edge-to-edge repair with TriClip. This system achieves the Edge-to-Edge repair (TEER) similarly to MitraClip, but it has certain features that make more patients amenable to such therapy.
Image 3. PASCAL and its delivery catheter
The PASCAL system consists of three main subsystems (Image 3): A deflectable guide catheter. A deflectable delivery system. A PASCAL device (implant).
The anatomical eligibility criteria are examined using transthoracic and intraesophageal echocardiography (Doppler ultrasound).
Conservative, surgical, and percutaneous treatment for mitral regurgitation shortly after acute myocardial infarction. Haberman D, Estévez-Loureiro R, Benito-Gonzalez T, Denti P, Arzamendi D, Adamo M, Freixa X, Nombela-Franco L, Villablanca P, Krivoshei L, Fam N, Spargias K, Czarnecki A, Pascual I, Praz F, Sudarsky D, Kerner A, Ninios V, Gennari M, Beeri R, Perl L, Wasserstrum Y, Danenberg H, Poles L, George J, Caneiro-Queija B, Scianna S, Moaraf I, Schiavi D, Scardino C, Corpataux N, Echarte-Morales J, Chrissoheris M, Fernández-Peregrina E, Di Pasquale M, Regueiro A, Vergara-Uzcategui C, Iñiguez-Romo A, Fernández-Vázquez F, Dvir D, Maisano F, Taramasso M, Shuvy M. Eur Heart J. 2021 Aug 31:ehab496.
2-Year Outcomes for Transcatheter Repair in Patients With Mitral Regurgitation From the CLASP Study. Szerlip M, Spargias KS, Makkar R, Kar S, Kipperman RM, O’Neill WW, Ng MKC, Smith RL, Fam NP, Rinaldi MJ, Raffel OC, Walters DL, Levisay J, Montorfano M, Latib A, Carroll JD, Nickenig G, Windecker S, Marcoff L, Cohen GN, Schäfer U, Webb JG, Lim DS. JACC Cardiovasc Interv. 2021 Jul 26;14(14):1538-1548.
Use of MitraClip for mitral valve repair in patients with acute mitral regurgitation following acute myocardial infarction: Effect of cardiogenic shock on outcomes (IREMMI Registry). Estévez-Loureiro R, Shuvy M, Taramasso M, Benito-Gonzalez T, Denti P, Arzamendi D, Adamo M, Freixa X, Villablanca P, Krivoshei L, Fam N, Spargias K, Czarnecki A, Haberman D, Agmon Y, Sudarsky D, Pascual I, Ninios V, Scianna S, Moaraf I, Schiavi D, Chrissoheris M, Beeri R, Kerner A, Fernández-Peregrina E, Di Pasquale M, Regueiro A, Poles L, Iñiguez-Romo A, Fernández-Vázquez F, Maisano F. Catheter Cardiovasc Interv. 2021 May 1;97(6):1259-1267.
Safety and Feasibility of MitraClip Implantation in Patients with Acute Mitral Regurgitation after Recent Myocardial Infarction and Severe Left Ventricle Dysfunction. Haberman D, Estévez-Loureiro R, Benito-Gonzalez T, Denti P, Arzamendi D, Adamo M, Freixa X, Nombela-Franco L, Villablanca P, Krivoshei L, Fam N, Spargias K, Czarnecki A, Pascual I, Praz F, Sudarsky D, Kerner A, Ninios V, Gennari M, Beeri R, Perl L, Danenberg H, Poles L, Shimoni S, Goland S, Caneiro-Queija B, Scianna S, Moaraf I, Schiavi D, Scardino C, Corpataux N, Echarte-Morales J, Chrissoheris M, Fernández-Peregrina E, Di Pasquale M, Regueiro A, Vergara-Uzcategui C, Iñiguez-Romo A, Fernández-Vázquez F, Dvir D, Taramasso M, Shuvy M. J Clin Med. 2021 Apr 22;10(9):1819
The effect of Remote Ischemic Preconditioning (RIPC) on myocardial injury and inflammation in patients with severe aortic valve stenosis undergoing Transcatheter Aortic Valve Replacement (TAVΙ). Halapas A, Kapelouzou A, Chrissoheris M, Pattakos G, Cokkinos DV, Spargias K. Hellenic J Cardiol. 2021 Feb 20:S1109-9666.
Transcatheter closure of paravalvular leak: Multicenter experience and follow-up. Kalogeras K, Ntalekou K, Aggeli K, Moldovan C, Katsianos E, Kalantzis C, Bei E, Mourmouris C, Spargias K, Chrissoheris M, Dardas P, Aznaouridis K, Tzifa A, Theofilogiannakos E, Siasos G, Tousoulis D, Vavuranakis M. Hellenic J Cardiol. 2021 Feb 19:S1109-9666(21)00015-4.
Emergency TAVI in a critically ill patient: A case report. Papadopoulos K, Chrissoheris M, Halapas A, Kourkoveli P, Ozden Tok O, Spargias K. Clin Case Rep. 2020 Dec 31;9(2):1024-1026.
Transcatheter Mitral Valve Replacement After Surgical Repair or Replacement: Comprehensive Midterm Evaluation of Valve-in-Valve and Valve-in-Ring Implantation From the VIVID Registry. Simonato M, Whisenant B, Ribeiro HB, Webb JG, Kornowski R, Guerrero M, Wijeysundera H, Søndergaard L, De Backer O, Villablanca P, Rihal C, Eleid M, Kempfert J, Unbehaun A, Erlebach M, Casselman F, Adam M, Montorfano M, Ancona M, Saia F, Ubben T, Meincke F, Napodano M, Codner P, Schofer J, Pelletier M, Cheung A, Shuvy M, Palma JH, Gaia DF, Duncan A, Hildick-Smith D, Veulemans V, Sinning JM, Arbel Y, Testa L, de Weger A, Eltchaninoff H, Hemery T, Landes U, Tchetche D, Dumonteil N, Rodés-Cabau J, Kim WK, Spargias K, Kourkoveli P, Ben-Yehuda O, Teles RC, Barbanti M, Fiorina C, Thukkani A, Mackensen GB, Jones N, Presbitero P, Petronio AS, Allali A, Champagnac D, Bleiziffer S, Rudolph T, Iadanza A, Salizzoni S, Agrifoglio M, Nombela-Franco L, Bonaros N, Kass M, Bruschi G, Amabile N, Chhatriwalla A, Messina A, Hirji SA, Andreas M, Welsh R, Schoels W, Hellig F, Windecker S, Stortecky S, Maisano F, Stone GW, Dvir D. Circulation. 2021 Jan 12;143(2):104-116.
1-Year Outcomes for Transcatheter Repair in Patients With Mitral Regurgitation From the CLASP Study. Webb JG, Hensey M, Szerlip M, Schäfer U, Cohen GN, Kar S, Makkar R, Kipperman RM, Spargias K, O’Neill WW, Ng MKC, Fam NP, Rinaldi MJ, Smith RL, Walters DL, Raffel CO, Levisay J, Latib A, Montorfano M, Marcoff L, Shrivastava M, Boone R, Gilmore S, Feldman TE, Lim DS. JACC Cardiovasc Interv. 2020 Oct 26;13(20):2344-2357.
Long-term outcomes after transcatheter aortic valve implantation in failed bioprosthetic valves. Bleiziffer S, Simonato M, Webb JG, Rodés-Cabau J, Pibarot P, Kornowski R, Windecker S, Erlebach M, Duncan A, Seiffert M, Unbehaun A, Frerker C, Conzelmann L, Wijeysundera H, Kim WK, Montorfano M, Latib A, Tchetche D, Allali A, Abdel-Wahab M, Orvin K, Stortecky S, Nissen H, Holzamer A, Urena M, Testa L, Agrifoglio M, Whisenant B, Sathananthan J, Napodano M, Landi A, Fiorina C, Zittermann A, Veulemans V, Sinning JM, Saia F, Brecker S, Presbitero P, De Backer O, Søndergaard L, Bruschi G, Franco LN, Petronio AS, Barbanti M, Cerillo A, Spargias K, Schofer J, Cohen M, Muñoz-Garcia A, Finkelstein A, Adam M, Serra V, Teles RC, Champagnac D, Iadanza A, Chodor P, Eggebrecht H, Welsh R, Caixeta A, Salizzoni S, Dager A, Auffret V, Cheema A, Ubben T, Ancona M, Rudolph T, Gummert J, Tseng E, Noble S, Bunc M, Roberts D, Kass M, Gupta A, Leon MB, Dvir D. Eur Heart J. 2020 Aug 1;41(29):2731-2742.
MitraClip and left ventricular reverse remodelling: a strain imaging study. Papadopoulos K, Ikonomidis I, Chrissoheris M, Chalapas A, Kourkoveli P, Parissis J, Spargias K. ESC Heart Fail. 2020 Aug;7(4):1409-1418.
Transcatheter mitral valve-in-ring implantation by the transfemoral approach: First experience in Greece. Chrissoheris MP, Halapas A, Papadopoulos K, Kourkoveli P, Nikolaou I, Bouboulis N, Pattakos G, Spargias K. Hellenic J Cardiol. 2020 Mar-Apr;61(2):135-136.
Novel Multiphase Assessment for Predicting Left Ventricular Outflow Tract Obstruction Before Transcatheter Mitral Valve Replacement. Meduri CU, Reardon MJ, Lim DS, Howard E, Dunnington G, Lee DP, Liang D, Gooley R, O’Hair D, Ng MK, Walton A, Spargias K, Blackman D, Coisne A, Hildick-Smith D, De Gouy M, Chenoweth S, Kar S, McCarthy PM, Piazza N, Qasam A, Martin RP, Leon MB, Mack MJ, Adams DH, Bapat V. JACC Cardiovasc Interv. 2019 Dec 9;12(23):2402-2412.
Contemporary Presentation and Management of Valvular Heart Disease: The EURObservational Research Programme Valvular Heart Disease II Survey. Iung B, Delgado V, Rosenhek R, Price S, Prendergast B, Wendler O, De Bonis M, Tribouilloy C, Evangelista A, Bogachev-Prokophiev A, Apor A, Ince H, Laroche C, Popescu BA, Piérard L, Haude M, Hindricks G, Ruschitzka F, Windecker S, Bax JJ, Maggioni A, Vahanian A; EORP VHD II Investigators. Circulation. 2019 Oct;140(14):1156-1169.
Haberman D, Taramasso M, Czarnecki A, Kerner A, Chrissoheris M, Spargias K, Poles L, Agmon Y, Scianna S, Beeri R, Lotan C, Maisano F, Shuvy M. Salvage MitraClip in severe secondary mitral regurgitation complicating acute myocardial infarction: data from a multicentre international study. Eur J Heart Fail. 2019 Sep;21(9):1161-1164. doi: 10.1002/ejhf.1565. Epub 2019 Aug 5.
Simonato M, Webb J, Bleiziffer S, Abdel-Wahab M, Wood D, Seiffert M, Schäfer U, Wöhrle J, Jochheim D, Woitek F, Latib A, Barbanti M, Spargias K, Kodali S, Jones T, Tchetche D, Coutinho R, Napodano M, Garcia S, Veulemans V, Siqueira D, Windecker S, Cerillo A, Kempfert J, Agrifoglio M, Bonaros N, Schoels W, Baumbach H, Schofer J, Gaia DF, Dvir D. Current Generation Balloon-Expandable Transcatheter Valve Positioning Strategies During Aortic Valve-in-Valve Procedures and Clinical Outcomes. JACC Cardiovasc Interv. 2019 Aug 26;12(16):1606-1617. doi: 10.1016/j.jcin.2019.05.057.
Asmarats L, Perlman G, Praz F, Hensey M, Chrissoheris MP, Philippon F, Ofek H, Ye J, Puri R, Pibarot P, Attinger A, Moss R, Bédard E, Moschovitis A, Reineke D, Lauck S, Blanke P, Leipsic J, Spargias K, Windecker S, Webb JG, Rodés-Cabau J. Long-Term Outcomes of the FORMA Transcatheter Tricuspid Valve Repair System for the Treatment of Severe Tricuspid Regurgitation: Insights From the First-in-Human Experience. JACC Cardiovasc Interv. 2019 Aug 12;12(15):1438-1447. doi: 10.1016/j.jcin.2019.04.038.
Lim DS, Kar S, Spargias K, Kipperman RM, O’Neill WW, Ng MKC, Fam NP, Walters DL, Webb JG, Smith RL, Rinaldi MJ, Latib A, Cohen GN, Schäfer U, Marcoff L, Vandrangi P, Verta P, Feldman TE. Transcatheter Valve Repair for Patients With Mitral Regurgitation: 30-Day Results of the CLASP Study. JACC Cardiovasc Interv. 2019 Jul 22;12(14):1369-1378. doi: 10.1016/j.jcin.2019.04.034. Epub 2019 Jun 26.
Duncan A, Moat N, Simonato M, de Weger A, Kempfert J, Eggebrecht H, Walton A, Hellig F, Kornowski R, Spargias K, Mendiz O, Makkar R, Guerrero M, Rihal C, George I, Don C, Iadanza A, Bapat V, Welsh R, Wijeysundera HC, Wood D, Sathananthan J, Danenberg H, Maisano F, Garcia S, Gafoor S, Nombela-Franco L, Cobiella J, Dvir D. Outcomes Following Transcatheter Aortic Valve Replacement for Degenerative Stentless Versus Stented Bioprostheses. JACC Cardiovasc Interv. 2019 Jul 8;12(13):1256-1263. doi: 10.1016/j.jcin.2019.02.036. Epub 2019 Jun 12.
Chrissoheris MP, Halapas A, Papadopoulos K, Kourkoveli P, Nikolaou I, Bouboulis N, Pattakos G, Spargias K. Transcatheter mitral valve-in-ring implantation by the transfemoral approach: First experience in Greece. Hellenic J Cardiol. 2019 Jun 5. pii: S1109-9666(19)30053-3. doi: 10.1016/j.hjc.2019.05.003. [Epub ahead of print]
Papadopoulos K, Chrissoheris M, Nikolaou I, Spargias K. Edge-to-edge mitral valve repair for acute mitral valve regurgitation due to papillary muscle rupture: a case report. Eur Heart J Case Rep. 2019 Feb 6;3(1):ytz001. doi: 10.1093/ehjcr/ytz001. eCollection 2019 Mar.
Pattakos G, Chrissoheris M, Halapas A, Papadopoulos K, Kourkoveli P, Bouboulis N, Pattakos S, Spargias K. Transcatheter Aortic Valve Replacement in a Patient With Dextrocardia and Situs Inversus Totalis. Ann Thorac Surg. 2019 Jan;107(1):e33-e35. doi: 10.1016/j.athoracsur.2018.05.041. Epub 2018 Aug 16.
McElhinney DB, Aboulhosn JA, Dvir D, Whisenant B, Zhang Y, Eicken A, Ribichini F, Tzifa A, Hainstock MR, Martin MH, Kornowski R, Schubert S, Latib A, Thomson JDR, Torres AJ, Meadows J, Delaney JW, Guerrero ME, Salizzoni S, El-Said H, Finkelstein A, George I, Gewillig M, Alvarez-Fuente M, Lamers L, Cheema AN, Kreutzer JN, Rudolph T, Hildick-Smith D, Cabalka AK; VIVID Registry. Mid-Term Valve-Related Outcomes After Transcatheter Tricuspid Valve-in-Valve or Valve-in-Ring Replacement. J Am Coll Cardiol. 2019 Jan 22;73(2):148-157. doi: 10.1016/j.jacc.2018.10.051.
Chrissoheris MP, Halapas A, Papadopoulos K, Spargias K. Transcatheter MitraClip implantation facilitated by transthoracic echocardiography. J Echocardiogr. 2018 Jun;16(2):91-92. doi: 10.1007/s12574-017-0358-0. Epub 2017 Nov 27.
Pibarot P, Simonato M, Barbanti M, Linke A, Kornowski R, Rudolph T, Spence M, Moat N, Aldea G, Mennuni M, Iadanza A, Amrane H, Gaia D, Kim WK, Napodano M, Baumbach H, Finkelstein A, Kobayashi J, Brecker S, Don C, Cerillo A, Unbehaun A, Attias D, Nejjari M, Jones N, Fiorina C, Tchetche D, Philippart R, Spargias K, Hernandez JM, Latib A, Dvir D. Impact of Pre-Existing Prosthesis-Patient Mismatch on Survival Following Aortic Valve-in-Valve Procedures. JACC Cardiovasc Interv. 2018 Jan 22;11(2):133-141. doi: 10.1016/j.jcin.2017.08.039.
Bapat V, Rajagopal V, Meduri C, Farivar RS, Walton A, Duffy SJ, Gooley R, Almeida A, Reardon MJ, Kleiman NS, Spargias K, Pattakos S, Ng MK, Wilson M, Adams DH, Leon M, Mack MJ, Chenoweth S, Sorajja P; Intrepid Global Pilot Study Investigators. Early Experience With New Transcatheter Mitral Valve Replacement. J Am Coll Cardiol. 2018 Jan 2;71(1):12-21. doi: 10.1016/j.jacc.2017.10.061. Epub 2017 Nov 16.
Taggart NW, Cabalka AK, Eicken A, Aboulhosn JA, Thomson JDR, Whisenant B, Bocks ML, Schubert S, Jones TK, Asnes JD, Fagan TE, Meadows J, Hoyer M, Martin MH, Ing FF, Turner DR, Latib A, Tzifa A, Windecker S, Goldstein BH, Delaney JW, Kuo JA, Foerster S, Gillespie M, Butera G, Shahanavaz S, Horlick E, Boudjemline Y, Dvir D, McElhinney DB; VIVID Registry. Outcomes of Transcatheter Tricuspid Valve-in-Valve Implantation in Patients With Ebstein Anomaly. Am J Cardiol. 2018 Jan 15;121(2):262-268. doi: 10.1016/j.amjcard.2017.10.017. Epub 2017 Oct 19.
Praz F, Spargias K, Chrissoheris M, et al. Compassionate use of the PASCAL transcatheter mitral valve repair system for patients with severe mitral regurgitation: a multicentre, prospective, observational, first-in-man study. Lancet. 2017;390:773-780
Kourkoveli P, Spargias K, Hahalis G. TAVR in 2017-What we know? What to expect? J Geriatr Cardiol. 2018 Jan;15(1):55-60. doi: 10.11909/j.issn.1671-5411.2018.01.005.
FORWARD Study Investigators (Spargias K) Clinical Outcomes With a Repositionable Self-Expanding Transcatheter Aortic Valve Prosthesis: The International FORWARD Study. J Am Coll Cardiol. 2017;70(7):845-853.
K Spargias, A Manginas, G Pavlides, M Khoury, G Stavridis, P Rellia, A Smirli, A Thanopoulos, M Balanika, S Polymeros, S Thomopoulou, G Athanassopoulos, G Karatasakis, R Mastorakou, S Lacoumenta, A Michalis, P Alivizatos, D Cokkinos. Transcatheter Aortic Valve Implantation: First Greek Experience. Hellenic J Cardiol 2008;49:397-407.
Spargias K, Chrissoheris M, Halapas A, Nikolaou J, Tsolakis A, Bouboulis N, Pattakos S. Percutaneous Mitral Valve Repair Using the Edge-to-Edge Technique: First Greek Experience. Hellenic J Cardiol 2012;53:343-51.
K Spargias, K Toutouzas, M Chrissoheris, Synetos A, Halapas A, Paizis I, Latsios G, Stahogianis K, Papametzelopoulos S, Zanos S, Pavlides G, Zacharoulis A, Antoniades A, Stefanadis C. The ATHENS TAVI Registry of newer generation transfemoral aortic valves. 30 day outcomes. Hellenic J Cardiol 2013;54:18-24.
Chrissoheris M, Ziakas A, Halapas A, Hadjimiltiades S, Styliadis I, Karvounis C, Nikolaou I, Spargias K. Acute Invasive Hemodynamic Effects of Transcatheter Aortic Valve Implantation. J Heart Valve Disease 2016, Mar;25(2):162-172.
K Spargias, A Halapas, M Chrissoheris, N Bouboulis, S Skardoutsos, J Nikolaou, A Tsolakis, C Mourmouris, S Pattakos. Transaortic Aortic Valve Replacement Using The Edwards Sapien-XT Valve And The Medtronic Corevalve. Initial Experience. Hellenic J Cardiol 2014;55:288-93.
M Chrissoheris, A Tsangaris, A Halapas, K Spargias. Transcatheter Aortic Valve Replacement in Patients with Prior Chest Irradiation and Severe Aortic Stenosis. J Radiol Oncology 2016, In press.
Spargias K, Dvir D. TAVR In Failed Bioprostheses. The Valve-In-Valve Approach. Cardiac Interv Today 2013; 45-8.
Chrissoheris M, Halapas A, Nikolaou I, Boumboulis N, Pattakos S, Spargias K. The Abbott Vascular MitraClip: Patient Selection and How to Obtain the Best Outcomes. Hellenic J Cardiol. 2015;56 Suppl A:31-8.
Tzikas A, Chrissoheris M, Halapas A, Spargias K. PorticoTM Transcatheter Heart Valve. Hellenic J Cardiol. 2015;56 Suppl A:15-9.
Halapas A, Chrissoheris M, Bouboulis N, Skardoutsos S, Nikolaou I, Pattakos S, Spargias K. The SAPIEN-XT and SAPIEN-3 Valves: How to Implant and Obtain the Best Outcomes. Hellenic J Cardiol. 2015;56 Suppl A:9-14.
Spargias K. The era of transcatheter valve therapy. Where are we? Hellenic J Cardiol. 2015;56 Suppl A:1-3.
Wendler O, Walther T, Schroefel H, Lange R, Treede H, Fusari M, Rubino P, Thomas M; SOURCE investigators (K Spargias). Transapical aortic valve implantation: mid-term outcome from the SOURCE registry. Eur J Cardiothorac Surg 2013;43:505-11.
Spargias K, Tzifa A, Chrissoheris M, Boumpoulis N, Nikolaou I, Pattakos E. Transapical closure of mitral prosthetic paravalvular leak. Hellenic J Cardiol 2013;54:397-400.
A Halapas, M Chrissoheris, I Nikolaou, N Bouboulis, K Spargias. Challenging Transfemoral Valve-in-Valve Implantation in a Degenerated Stentless Bioprosthetic Aortic Valve. J Invasive Cardiol 2015;56:347-50
Papavasileiou LP, Halapas A, Chrisocheris M, Bellos K, Bouboulis N, Pattakos S, Spargias K, Apostolopoulos T. Sudden Death After ranscatheter Aortic Valve Implantation. Are Bradyarrhythmias Always The Cause? JAFIB. 2015;8:39-41.
K Spargias, G Dangas. Percutaneous Treatment of Left Side Cardiac Valves. C Tamburino and GP Ussia, Ed. Springer-Verlag, 2010, Milan, Italy.
Chrissoheris M, Spargias K. “Balloon Aortic Valvuloplasty” in Textbook: Cardiac Valvular Medicine. Rajamannan NM (Ed.). Springer-Verlag, 2013, London, UK.
Spargias K.
Percutaneous aortic valve implantation: present and future. Controlled clinical application before the results of randomized trials. Hellenic J Cardiol 2009;50:237-44.K Spargias, M Gyöngyösi, R Hemetsberger, A Posa, N Pavo, IJ Pavo, K Huber, Z Petrasi, O Petnehazy, R Pogge von Strandmann, SR Stock, D Glogar, G Maurer, NM Rajamannan. Valvuloplasty with a Paclitaxel-Eluting Balloon Prevents Restenosis in an Experimental Model of Aortic Stenosis. J Heart Valve Disease 2014 Jul;23(4):484-91.
Spargias K, Polymeros S, Dimopoulos A, Manginas A, Pavlides G, Balanika M, Smirli A, Stavridis G, Dangas G, Cokkinos DV. The predictive value and evolution of N-Terminal Pro-B-Type Natriuretic Peptide levels following Transcutaneous Aortic Valve Implantation. J Interv Cardiol 2011;24:462-9.
Balanika M, Smyrli A, Samanidis G, Spargias K, Stavridis G, Karavolias G, Khoury M, Voudris V, Lacoumenta S. Anesthetic Management of Patients Undergoing Transcatheter Aortic Valve Implantation. J Cardiothorac Vasc Anesth 2013 Dec 5. [Epub ahead of print]
Spargias K, Alexopoulos E, Thomopoulou S, Dimopoulos A, Manginas A, Pavlides G, Voudris V, Karatassakis G, Athanassopoulos G, Cokkinos DV. Effect of balloon valvuloplasty in patients with severe aortic stenosis on levels of N-terminal pro-B-type natriuretic peptide. Am J Cardiol 2009;104:846-849.
Dvir D, Assali A, Spargias K, Kornowski R. Percutaneous aortic valve implantation in patients with coronary artery disease: review of therapeutic strategies. J Invasive Cardiol 2009;21:E237-41.
Chrissoheris M, Ferti A, Spargias K. Early prosthetic valve endocarditis complicating repeated attempts at CoreValve implantation. J Invasive Cardiol 2011;23:E291-2.
Spargias K, Milewski K, Debinski M, Buszman PP, Cokkinos DV, Pogge R, Buszman P. Drug delivery at the aortic valve tissues of healthy domestic pigs with a paclitaxel eluting valvuloplasty balloon. J Interv Cardiol 2009;22:291-8.
Wilczek K, Chodór P, Przybylski R, Krasoń M, Niklewski T, Nadziakiewicz P, Głowacki J, Kusa J, Goddyn D, Spargias K, Zembala M. First in Poland transcatheter, transfemoral aortic valve implantation in elderly symptomatic high-risk patient with aortic stenosis-novel Zabrze experience. Kardiol Pol 2009;67:219-23.
Kolettis TN, Spargias K, Stavridis GT. Combined transapical aortic valve implantation with coronary artery bypass grafting in a young patient with porcelain aorta. Hellenic J Cardiol 2009;50:79-82.
Trendafilova D , Jorgova J, Simeonov P. Acknowledgement to Dr K Spargias. First Transfemoral Aortic Valve Implantation In Bulgaria. The Internet Journal of Cardiology 2010;9:2.
Director of Transcatheter Heart Valves Department is Konstatinos Spargias
Attending Physicians
Stratos Pattakos, DirectorGrigorios Pattakos, Αssoc. Director
Michael Chrissoheris, Αssoc. DirectorNikolaos Mpoumpoulis, Attending Physician
Panagiota Kourkoveli, Attending Physician
Dionisios Aravantinos, Attending Physician
Kyriakos Katsianos, Attending Phygsician
Konstantinos Stathogiannis, Attending Physician
Transcatheter aortic valve replacement/implantation (SAPIEN Ultra, Edwards Lifesciences; Evolut PRO, Medtronic; ACURATE neo2, Boston Scientific; Navitor, Abbott; JenaValve)
Transcatheter mitral valve regurgitation repair (MitraClip, PASCAL)
Transcatheter mitral valve replacement (SAPIEN Ultra, Edwards Lifesciences and various devices as part of research)
Transcatheter tricuspid valve regurgitation repair (TriClip, PASCAL)
Transcatheter tricuspid valve replacement (SAPIEN Ultra, Edwards Lifesciences and various devices as part of research)
Transcatheter paravalvular leak closure
Transcatheter PFO and ASD closure
Cardiac valvuloplasty
Coronary angiography
Percutaneous coronary artery revascularization (angioplasty-stent)
Cardiac catheterizations
Cardiac biopsy
Pericardiocentesis
Alcohol septal ablation
Pulmonary hypertension reactivity testing
Aortic Valve Stenosis
The aortic valve is one of the 4 valves of the heart and it ensures blood flow from the heart to the rest of the body. The normal aortic valve opening is 3-4 cm2. The stenosis of the valve is considered significant when the opening is reduced to less than 1 cm2.
The degenerative mechanism that causes aortic valve stenosis (narrowing) is dynamic and evolving. The main features of this process are gradual stiffening and thickening of the valve with calcium deposits that progressively limit leaflet mobility and eventually narrow the valve. Approximately 5% of the elderly population (>75 years) have developed at least moderate aortic valve stenosis.
When the condition becomes severe, it develops rapidly and the prognosis is poor, with high morbidity and hassles for the patients and their families.
The most common symptoms of aortic valve stenosis are shortness of breath, angina and dizziness, up to syncopal attacks. After the onset of symptoms, average patient survival drops drastically.
Treatment
Pharmaceutical treatment can relieve some of the symptoms temporarily, but it does not affect or treat the valve condition. The only treatment is valve replacement, which could only be performed with open surgery until a few years ago. However, many patients are already of an advanced age when the symptoms appear and often suffer from other conditions that greatly increase the risk of conventional cardiac surgery, or even prohibit it. Therefore, surgical replacement is considered too risky in these patients and they often do not undergo surgery (it is estimated that 1 in every 2 patients who must undergo conventional surgical replacement of aortic valve is rejected). Prognosis in these patients is poor and morbidity long and painful.
Transcatheter Aortic Valve Replacement (Implantation): TAVR or TAVI is being successfully performed since 2007, initially on patients who could not have been treated in the past. Since 2011, TAVR has also been approved and is successfully performed on patients who could have undergone surgical replacement, but were considered high risk. With low single-digit mortality rates and fast recovery and return to daily activities, the benefits of this method are obvious.
In 2016, the results of the first clinical trials showed better survival with TAVR compared to the surgical replacement, even in lower (moderate) risk patients, and the use of the method has since been extended to these patients as well. In 2018, newer clinical trials produced similar results for the TAVR method in low-risk patients (essentially for all patients now). As a result, the method was recently (2019) approved and indicated by the FDA for application on essentially all patients with aortic valve stenosis.
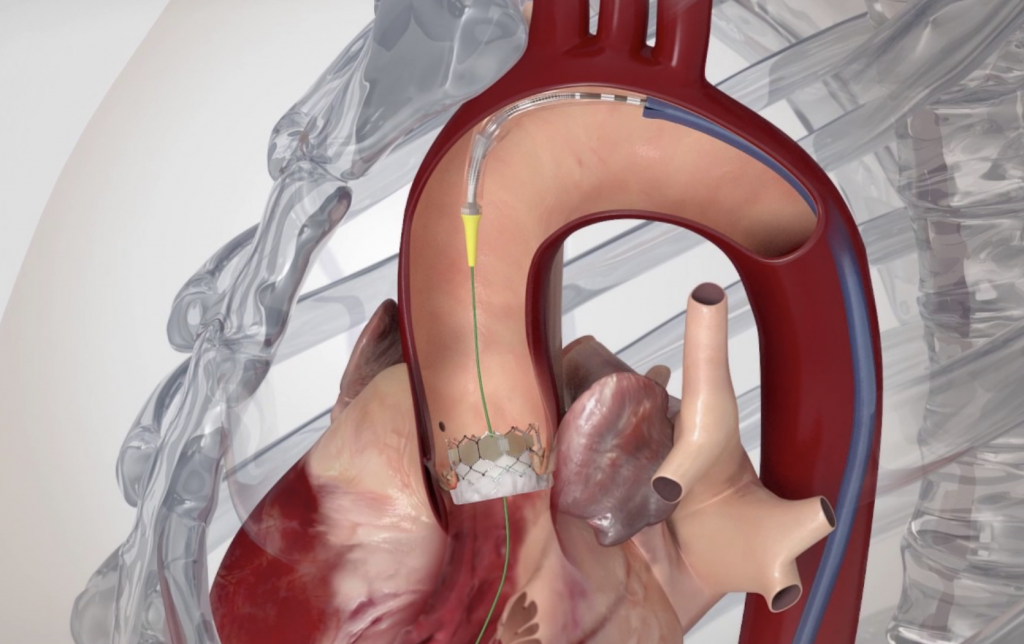
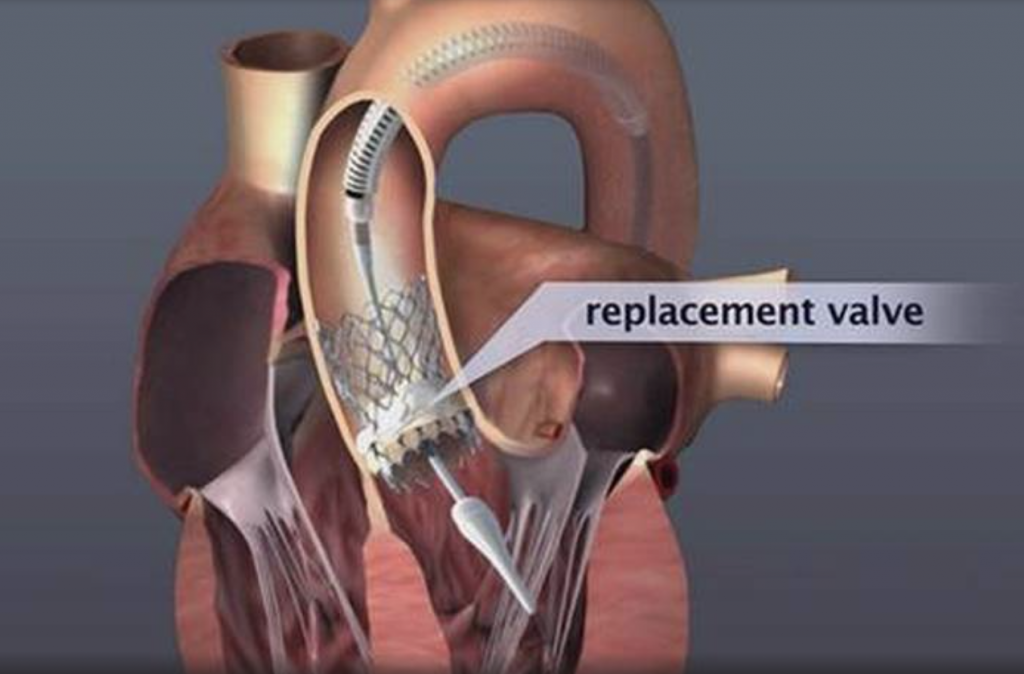
Image 1. Illustration of implanted transcatheter valves in the position of the aortic valve
The Studies
The Transcatheter Aortic Valve Replacement is the most researched valve replacement method, as well as one of the most researched treatments in Cardiology and Cardiac Surgery in general.
The results of the randomized PARTNER-B study were announced in 2010, when it was found that the transcatheter method for treating aortic valve stenosis is changing the natural history of the disease and improves survival. Older patients with coexisting conditions, who would generally be treated conservatively due to the forbiddingly high surgical risk, underwent randomized transfemoral valve implantation and showed remarkable improvement of symptoms, reduced need for hospitalization by 54% and reduced morbidity by 45% as early as the first year. Consequently, the method was recognized for this patient population, who would otherwise be doomed to severe morbidity and very poor prognosis. It is estimated that there are at least 1,000 such patients in Greece (per year) who could benefit from the transcatheter approach.
The results of the randomized PARTNER-A study were announced in March 2011. PARTNER-A is significant in that it randomized high-risk patients (Logistic Euroscore ≥ 15%), who, however, were not rejected for surgical treatment. 30-day morbidity for the transcatheter procedure (3.4%) was almost 50% lower compared to the corresponding surgical replacement (6.7%), proving that they are at least equivalent treatments. Survival in the first year was 2.6% higher for the transcatheter method. Especially with regard to the transfemoral implantation, 30-day survival was significantly higher compared to the surgical replacement.
The results of PARTNER-A provided the first comparative assessment of the transcatheter implantation compared to the surgical replacement of the aortic valve, demonstrating that even high-risk patients who can undergo surgery enjoy just as good results with transcatheter implantation, and with all the benefits of a less invasive method. This population is estimated at around 25% of patients who undergo surgical replacement to date (patients with a Logistic Euroscore ≥ 15%) (approximately 300 patients/year in Greece, on top of the ~1000 deemed inoperable).
The first randomized clinical study that proved the superiority of the transcatheter method over the conventional surgical replacement was announced in the spring of 2014 (CoreValve US Pivotal High Risk Study). In this study, 795 high-risk patients (Logistic Euroscore average of 18%) who were eligible for conventional surgery were randomized to transcatheter or surgical replacement. The morbidity of those who underwent the transfemoral method was significantly lower both at 1 month (3.3% for the transcatheter, 4.5% for the surgical) and at 1 year (14.2% for the transcatheter, 19.1% for the surgical).
The results of the latest generation SAPIEN 3 valve by Edwards Lifesciences on 1,655 high and medium surgical risk patients were announced in the beginning of 2016. This study showed the lowest 30-day morbidity rates to date, i.e. 1.6% for the transfemoral procedure in high-risk patients and 1.1% in medium-risk patients.
The results of the PARTNER 2 study, which randomized 2,032 medium-risk patients (STS score 4%-8%) to transcatheter or surgical aortic valve replacement, were also announced in the beginning of 2016. The patients who underwent transfemoral valve implantation had better early survival as opposed to those who underwent conventional surgical replacement. The results of these studies provided the indication and established the transcatheter replacement in medium-risk patients (around 25% of patients undergoing surgical replacement up until that time).
The results of the PARTNER 3 and the EVOLUT Low Risk Trials were both announced at the beginning of 2019. They included 2,468 low-risk patients (STS < 4%) and during the 2-year follow-up, they showed that not only is TAVR safe and effective in these patients as well, but the incidence of death or stroke was halved compared to the conventional surgical replacement (PARTNER 3 and EVOLUT Low Risk Trials combined: 2.6% vs. 5.2%). In practical terms this means that if 200 such patients undergo TAVR as opposed to conventional surgery, 5 more will return for the 2-year follow-up alive and without having suffered a stroke. These are concrete results and will unavoidably lead to the generalized application of the TAVR in patients with aortic valve stenosis.
As of 2007, there has been wide clinical acceptance of the method (initially in Europe, followed by the USA and Japan) and the number of procedures performed followed a geometric progression, with an 40%-80% rise annually. More than 700,000 implantations have been performed to date (09/2019) globally (3,500 in Greece). In many countries, transcatheter implantations have surpassed the surgical ones. In most western European countries, more than 100 (and up to 300) transcatheter implantations are performed per 1 million population.
With regard to the clinical results in everyday practice, the announcements from the most recent register recordings and the studies show that 30-day morbidity in high/medium-risk patients who undergo transfemoral transcatheter replacement is around 2%-4%, while it is 1% in low-risk patients. The recorded 30-day morbidities for transthoracic and transaortic implantations are around 3%-8%.
Transcatheter implantations of all valve types currently available (SAPIEN 3, Edwards Lifesciences; Evolut PRO, Medtronic; Portico, Abbott; ACURATE neo and LOTUS Edge, Boston Scientific) are performed at HYGEIA Hospital, using all possible implantation methods (transfemoral, transaortic, transthoracic, subclavian route etc.). The Department staff’s experience exceeds 1,400 procedures, while 30-day morbidity of transfemoral procedures has been < 2%.
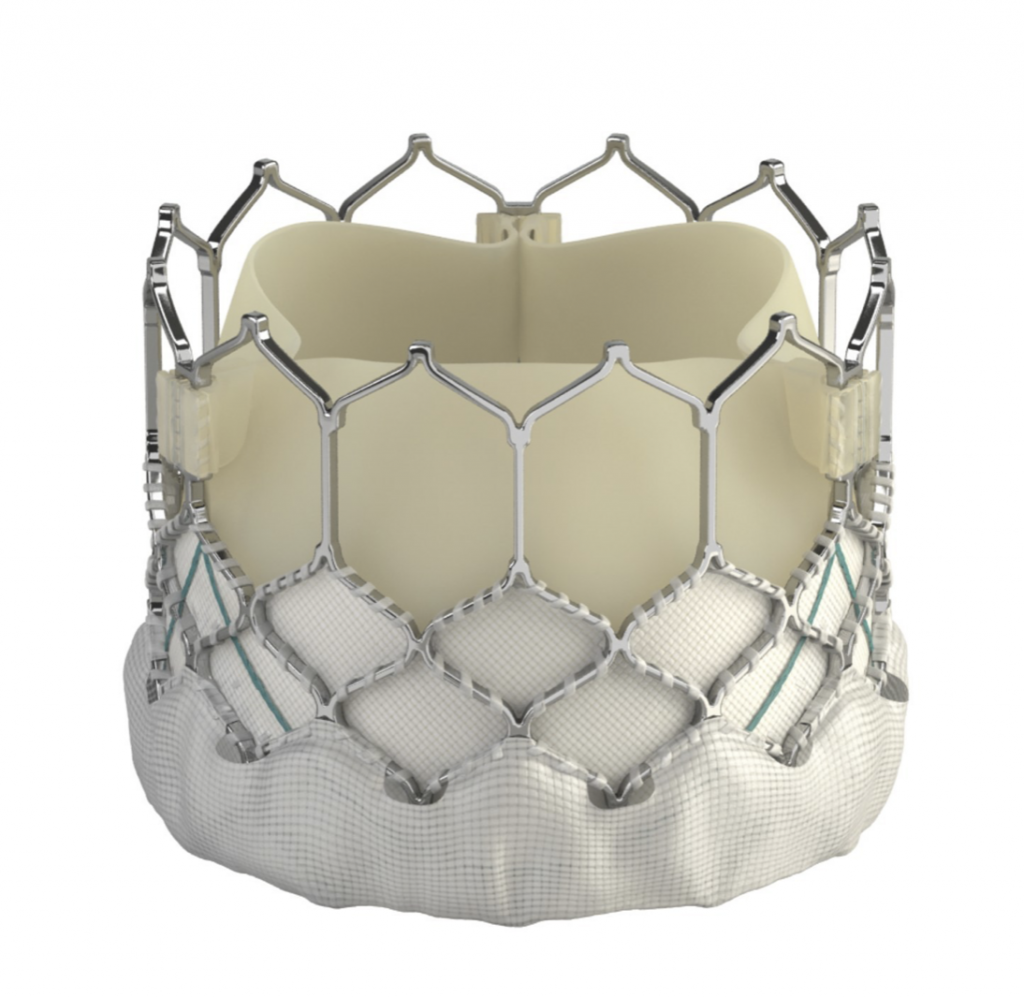
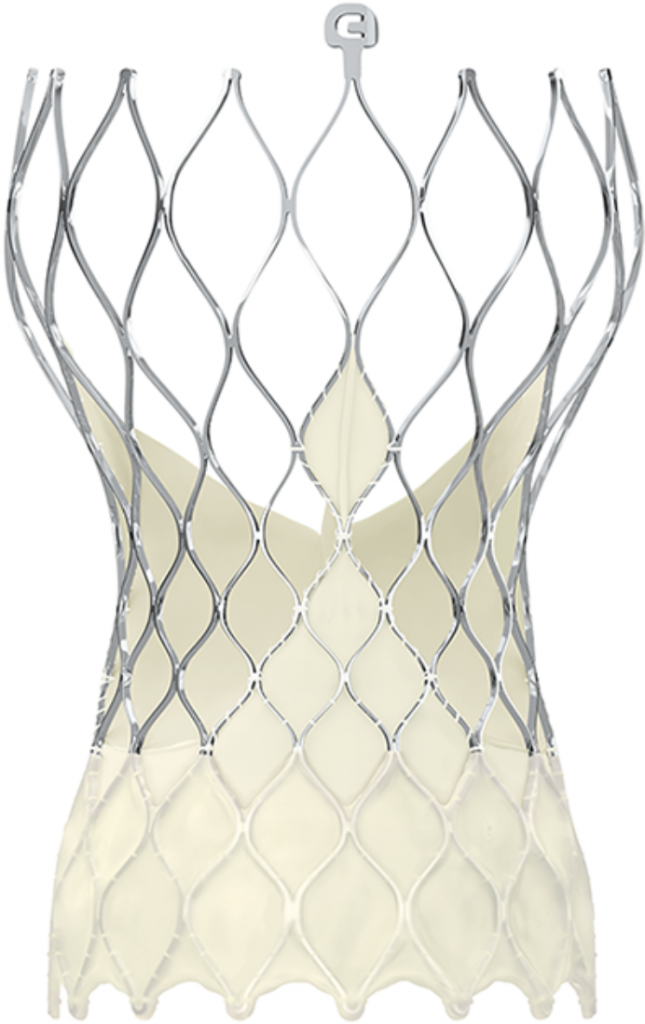
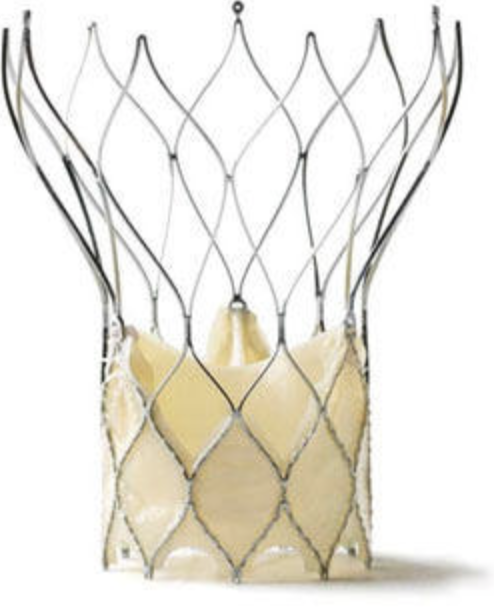


Image 2: The SAPIEN 3 (Edwards Lifesciences), Evolut PRO (Medtronic), Portico (Abbott), ACURATE neo and LOTUS Edge (Boston Scientific) transcatheter valves (from top left to bottom right)
Transcatheter Heart Valve Resilience
There is plenty of detailed follow-up information on many patients who carry transcatheter valves in the last 10 years and there have been no concerns as to their long-term resilience and functionality. Besides, they are manufactured by the same companies that manufacture surgical valves and undergo the same rigorous bench testing. It is expected that they will continue to operate well for just as long (i.e. 12-16 years). However, even if they malfunction, this will happen gradually and it will be possible to replace them with new transcatheter valves that will be implanted in the same simple manner. This is already being done on patients with malfunction of older surgically placed bioprosthetic valves, however, this time these patients are treated with the transcatheter method rather than undergoing a new surgical procedure.
Aortic Valve Regurgitation
Although it is rarer than aortic valve stenosis, aortic valve regurgitation causes the same serious problems and treatment is once again replacement of the faulty valve. Provided it can be performed, transcatheter replacement is the best choice for high-risk patients.
The Procedure
Transfemoral procedure
The procedure is performed through the femoral artery, similarly to a coronary angiography. Strict antisepsis measures are in place in the HYGEIA Hospital Hybrid Operating Room, where the procedure is performed. General anesthesia and intubation are not required, but patients are placed under sedation, so that they remain calm and can cooperate. The procedure usually lasts less than 30 minutes, but patients remain in the operating room for about 90 minutes total (preparation upon arrival and departure). The entry point of the valve into the femoral artery is sealed externally, while there is no incision on the skin.
Patients remain on bed rest for the first night postoperatively, at the Intensive Care Unit or High Dependency Unit, and then they are fully mobile and remain hospitalized in a simple ward for another 1-3 days. In the vast majority of patients (> 95%), the femoral arteries are suitable for valve implantation. It is generally accepted that transfemoral implantations are preferable, as they are less invasive and carry a lower risk.
Transaortic and transapical (transthoracic) procedure
Following the advancement and improvement of transcatheter valves, transaortic and transapical implantations are rarely performed these days (less than 5% of patients), and only when the femoral arteries are deemed unsuitable for transfemoral implantation (due to small size, stenosis, etc.). The procedure is performed under general anesthesia and the valve is implanted either through the aorta (transaortic), through a small incision between the ribs at the top of the chest, or directly through the heart (transapical), through a small incision between the ribs on the left, under the nipple. The procedure usually lasts less than 1 hour, but patients remain in the operating room for about 2 hours total (preparation upon arrival and departure). The incision on the skin between the ribs is around 3-5 cm long and is sutured closed. Patients remain on bed rest in the Intensive Care Unit for the first day postoperatively and then they are hospitalized in a simple ward for another 2-3 days.
These patients also enjoy the key benefits of the method, avoiding a sternotomy and extracorporeal circulation. The SAPIEN 3 and ACURATE neo valves may be implanted transaortically and transapically, the Evolut R and Portico transaortically, and the JenaValve transapically.
Eligibility testing
A patient’s eligibility is assessed through a series of tests as an outpatient, and rarely is 1-day hospitalization required. The tests include coronary angiography to check the heart arteries (invasive or bloodless with CT), cardiac CT, CT angiography of the limbs to determine their suitability for valve insertion, echocardiography, blood tests and any other exams deemed necessary, depending on each patient. Patients are also evaluated by the entire medical team that will participate in the procedure (cardiologists, cardiac surgeons, anesthesiologists, vascular surgeons, etc., i.e. the Heart Team). After testing, they decide on: the implantation method (transfemoral, transaortic, transapical, etc.), most suitable valve type (for anatomical and other reasons, one type of valve is often preferable to another in each patient) and suitable valve size.
Through this procedure, it is also possible to manage any significant stenoses encountered in the coronary arteries using angioplasty and stents. Finally, palliative valvuloplasty (valve opening with balloon) may be performed on gravely ill patients, so they may undergo valve implantation at a later date.
Mitral Valve Regurgitation
It is the most frequent valve disease and it is estimated that 8%-10% of people aged over 75 may develop at least moderate mitral valve regurgitation. The inability of this valve to close tightly results in part of the blood, which was to be channeled to the entire body through the aorta, flowing back into the lungs.
Image 1. Illustration of Mitral valve regurgitation (insufficiency)
Severe mitral valve regurgitation causes heart failure symptoms (fatigue and shortness of breath during natural fatigue, up to acute pulmonary edema) and predisposition for developing atrial fibrillation (arrhythmia) and stroke.
Mitral valve regurgitation is distinguished into functional (most prevalent, usually due to coronary disease or dilated cardiomyopathy) and organic (often degenerative, which causes leaflet prolapse). Pharmaceutical treatment may manage and temporarily improve some of the symptoms caused by severe mitral valve regurgitation. However, given that the problem is primarily mechanical, it cannot treat it.
Surgical treatment of the functional regurgitation of the mitral valve is attempted only when the patient also suffers from coronary artery disease and must undergo aortocoronary bypass surgery for precisely this reason. Otherwise the benefit is unconfirmed and the risk of the procedure high. This is also the reason why patients rarely undergo surgical procedure just to treat the functional valve regurgitation. Transcatheter repair is most applicable to them.
Surgical treatment of organic valve regurgitation usually involves open or endoscopic surgical repair. However, at times, this is not possible, in which case, it is replaced by a prosthetic valve. Nevertheless, there are many such patients who do not eventually undergo surgical treatment because they are deemed high risk for various reasons. Transcatheter repair is also applicable to them.
The percutaneous repair method used today is called Transcatheter Edge-to-Edge Repair (TEER) and is accomplished with the use of two available devices: MitraClip and PASCAL.
MitraClip: Transcatheter mitral valve regurgitation repair system
The MitraClip system (Abbott Vascular) is based on the transcatheter placement of one or more clips in the mitral valve and aims to reduce or even eliminate the regurgitation. This minimally invasive mitral valve repair mimics the open surgery method by creating a double orifice (Alfieri method) and increases the treatment choices available to patients who suffer from severe valve regurgitation.
Image 2. MitraClip and its delivery catheter
The MitraClip device places a clip in the mitral valve, without opening the patient’s chest and without incisions. The guide catheter is introduced through the femoral vein to reach the heart, similarly to a coronary angiography. The procedure is performed under general anesthesia, without using a heart-lung machine (extracorporeal circulation) and without a sternotomy. Hospitalization is two to three days postoperatively.
It has been proven to reduce the symptoms of heart failure, improve the quality of life and prolongs survival in select patients with functional mitral valve regurgitation. The method is very effective and has extremely low risk for complications.
The MitraClip system consists of three main subsystems (Image 2): A steerable guide catheter. A clip delivery system. A MitraClip device (implant).
The MitraClip is the first transcatheter mitral valve regurgitation repair system to receive the European CE certification in 2008 and be approved by the FDA in 2013, while more than 150,000 procedures have been performed globally to date.
The COAPT clinical trial, reported in 2018, included 603 patients with functional mitral valve regurgitation and compared the MitraClip with the conservative conventional treatment. It is extremely important that treatment using the MitraClip method showed significant and major reduction in morbidity, resulting in the US FDA extending the treatment indications in 2019.
In Greece, these procedures were performed for the first time by the HYGEIA Hospital Transcatheter Heart Valve Department in October 2011 and were met with great success. The program has been successfully continuing since then and more than 350 clips have been implanted.
PASCAL: Transcatheter mitral valve regurgitation repair system
The clinical application of the transcatheter mitral valve regurgitation repair using the PASCAL system by Edwards Lifesciences was approved in February 2019. This system achieves the Edge-to-Edge repair (TEER) similarly to MitraClip, but it has certain features that make more patients amenable to such therapy.
The HYGEIA Hospital Transcatheter Heart Valve Department was among the main investigators of the CLASP trial, which led to the PASCAL system receiving the CE mark due to its excellent results. The PASCAL system is currently used for the transcatheter repair of the mitral valve. The technique is similar to the one used for the MitraClip system, which is being successfully applied at HYGEIA for many years. The first commercial application in Greece was at the HYGEIA Hospital Transcatheter Heart Valve Department in 2019.
Image 3. PASCAL and its delivery catheter
The PASCAL system consists of three main subsystems (Image 3): A deflectable guide catheter. A deflectable delivery system. A PASCAL device (implant).
Possible treatment indications for MitraClip/PASCAL
The implantation of the clip may significantly reduce or even eliminate mitral valve regurgitation in the following cases:
-Significant functional mitral valve regurgitation that does not require surgical treatment of coexisting coronary artery disease.
-Significant organic mitral valve regurgitation in high surgical risk patients.
The anatomical eligibility criteria are examined using transthoracic and intraesophageal echocardiography (Doppler ultrasound).
HYGEIA Transcatheter Heart Valve Department is or has been official worldwide training center for Edwards’ SAPIEN 3 valves, Medtronic’s Evolut R valves, and its medical staff are Edwards, Medtronic, and Mitraclip/PASCAL Proctors.
The Department is continuously organizing the first Specialized Conference on Transcutaneous Cardiac Valve Diseases (www.thvgreece.com) in Greece since 2012 with the participation of Greek and foreign scientists and distinguished MDs for the purpose of continuously informing and educating about the latest developments in this field.
The Department launched a new monthly educational activity entitled “TAVI Monthly Rounds” since February 2021 (https://www.livemedia.gr/tavirounds21)
10th Transcatheter Heart Valves Greece 2021 (https://webtv.thvgreece.com/9thv)
8th Conference on Transcatheter Cardiac Valve 2019
7th Conference on Transcatheter Cardiac Valve 2018
6th Conference on Transcatheter Cardiac Valve 2017
5th Conference on Transcatheter Cardiac Valve 2016
4th Conference on Transcatheter Cardiac Valve 2015
3rd Conference on Transcatheter Cardiac Valve 2014
2nd Conference on Transcatheter Cardiac Valve 2013
1st Conference on Percutaneous Treatment of Cardiac Valves 2012
- Haberman D, Taramasso M, Czarnecki A, Kerner A, Chrissoheris M, Spargias K, Poles L, Agmon Y, Scianna S, Beeri R, Lotan C, Maisano F, Shuvy M. Salvage MitraClip in severe secondary mitral regurgitation complicating acute myocardial infarction: data from a multicentre international study. Eur J Heart Fail. 2019 Sep;21(9):1161-1164. doi: 10.1002/ejhf.1565. Epub 2019 Aug 5.
- Simonato M, Webb J, Bleiziffer S, Abdel-Wahab M, Wood D, Seiffert M, Schäfer U, Wöhrle J, Jochheim D, Woitek F, Latib A, Barbanti M, Spargias K, Kodali S, Jones T, Tchetche D, Coutinho R, Napodano M, Garcia S, Veulemans V, Siqueira D, Windecker S, Cerillo A, Kempfert J, Agrifoglio M, Bonaros N, Schoels W, Baumbach H, Schofer J, Gaia DF, Dvir D. Current Generation Balloon-Expandable Transcatheter Valve Positioning Strategies During Aortic Valve-in-Valve Procedures and Clinical Outcomes. JACC Cardiovasc Interv. 2019 Aug 26;12(16):1606-1617. doi: 10.1016/j.jcin.2019.05.057.
- Asmarats L, Perlman G, Praz F, Hensey M, Chrissoheris MP, Philippon F, Ofek H, Ye J, Puri R, Pibarot P, Attinger A, Moss R, Bédard E, Moschovitis A, Reineke D, Lauck S, Blanke P, Leipsic J, Spargias K, Windecker S, Webb JG, Rodés-Cabau J. Long-Term Outcomes of the FORMA Transcatheter Tricuspid Valve Repair System for the Treatment of Severe Tricuspid Regurgitation: Insights From the First-in-Human Experience. JACC Cardiovasc Interv. 2019 Aug 12;12(15):1438-1447. doi: 10.1016/j.jcin.2019.04.038.
- Lim DS, Kar S, Spargias K, Kipperman RM, O’Neill WW, Ng MKC, Fam NP, Walters DL, Webb JG, Smith RL, Rinaldi MJ, Latib A, Cohen GN, Schäfer U, Marcoff L, Vandrangi P, Verta P, Feldman TE. Transcatheter Valve Repair for Patients With Mitral Regurgitation: 30-Day Results of the CLASP Study. JACC Cardiovasc Interv. 2019 Jul 22;12(14):1369-1378. doi: 10.1016/j.jcin.2019.04.034. Epub 2019 Jun 26.
- Duncan A, Moat N, Simonato M, de Weger A, Kempfert J, Eggebrecht H, Walton A, Hellig F, Kornowski R, Spargias K, Mendiz O, Makkar R, Guerrero M, Rihal C, George I, Don C, Iadanza A, Bapat V, Welsh R, Wijeysundera HC, Wood D, Sathananthan J, Danenberg H, Maisano F, Garcia S, Gafoor S, Nombela-Franco L, Cobiella J, Dvir D. Outcomes Following Transcatheter Aortic Valve Replacement for Degenerative Stentless Versus Stented Bioprostheses. JACC Cardiovasc Interv. 2019 Jul 8;12(13):1256-1263. doi: 10.1016/j.jcin.2019.02.036. Epub 2019 Jun 12.
- Chrissoheris MP, Halapas A, Papadopoulos K, Kourkoveli P, Nikolaou I, Bouboulis N, Pattakos G, Spargias K. Transcatheter mitral valve-in-ring implantation by the transfemoral approach: First experience in Greece. Hellenic J Cardiol. 2019 Jun 5. pii: S1109-9666(19)30053-3. doi: 10.1016/j.hjc.2019.05.003. [Epub ahead of print]
- Papadopoulos K, Chrissoheris M, Nikolaou I, Spargias K. Edge-to-edge mitral valve repair for acute mitral valve regurgitation due to papillary muscle rupture: a case report. Eur Heart J Case Rep. 2019 Feb 6;3(1):ytz001. doi: 10.1093/ehjcr/ytz001. eCollection 2019 Mar.
- Pattakos G, Chrissoheris M, Halapas A, Papadopoulos K, Kourkoveli P, Bouboulis N, Pattakos S, Spargias K. Transcatheter Aortic Valve Replacement in a Patient With Dextrocardia and Situs Inversus Totalis. Ann Thorac Surg. 2019 Jan;107(1):e33-e35. doi: 10.1016/j.athoracsur.2018.05.041. Epub 2018 Aug 16.
- McElhinney DB, Aboulhosn JA, Dvir D, Whisenant B, Zhang Y, Eicken A, Ribichini F, Tzifa A, Hainstock MR, Martin MH, Kornowski R, Schubert S, Latib A, Thomson JDR, Torres AJ, Meadows J, Delaney JW, Guerrero ME, Salizzoni S, El-Said H, Finkelstein A, George I, Gewillig M, Alvarez-Fuente M, Lamers L, Cheema AN, Kreutzer JN, Rudolph T, Hildick-Smith D, Cabalka AK; VIVID Registry. Mid-Term Valve-Related Outcomes After Transcatheter Tricuspid Valve-in-Valve or Valve-in-Ring Replacement. J Am Coll Cardiol. 2019 Jan 22;73(2):148-157. doi: 10.1016/j.jacc.2018.10.051.
- Chrissoheris MP, Halapas A, Papadopoulos K, Spargias K. Transcatheter MitraClip implantation facilitated by transthoracic echocardiography. J Echocardiogr. 2018 Jun;16(2):91-92. doi: 10.1007/s12574-017-0358-0. Epub 2017 Nov 27.
- Pibarot P, Simonato M, Barbanti M, Linke A, Kornowski R, Rudolph T, Spence M, Moat N, Aldea G, Mennuni M, Iadanza A, Amrane H, Gaia D, Kim WK, Napodano M, Baumbach H, Finkelstein A, Kobayashi J, Brecker S, Don C, Cerillo A, Unbehaun A, Attias D, Nejjari M, Jones N, Fiorina C, Tchetche D, Philippart R, Spargias K, Hernandez JM, Latib A, Dvir D. Impact of Pre-Existing Prosthesis-Patient Mismatch on Survival Following Aortic Valve-in-Valve Procedures. JACC Cardiovasc Interv. 2018 Jan 22;11(2):133-141. doi: 10.1016/j.jcin.2017.08.039.
- Bapat V, Rajagopal V, Meduri C, Farivar RS, Walton A, Duffy SJ, Gooley R, Almeida A, Reardon MJ, Kleiman NS, Spargias K, Pattakos S, Ng MK, Wilson M, Adams DH, Leon M, Mack MJ, Chenoweth S, Sorajja P; Intrepid Global Pilot Study Investigators. Early Experience With New Transcatheter Mitral Valve Replacement. J Am Coll Cardiol. 2018 Jan 2;71(1):12-21. doi: 10.1016/j.jacc.2017.10.061. Epub 2017 Nov 16.
- Taggart NW, Cabalka AK, Eicken A, Aboulhosn JA, Thomson JDR, Whisenant B, Bocks ML, Schubert S, Jones TK, Asnes JD, Fagan TE, Meadows J, Hoyer M, Martin MH, Ing FF, Turner DR, Latib A, Tzifa A, Windecker S, Goldstein BH, Delaney JW, Kuo JA, Foerster S, Gillespie M, Butera G, Shahanavaz S, Horlick E, Boudjemline Y, Dvir D, McElhinney DB; VIVID Registry. Outcomes of Transcatheter Tricuspid Valve-in-Valve Implantation in Patients With Ebstein Anomaly. Am J Cardiol. 2018 Jan 15;121(2):262-268. doi: 10.1016/j.amjcard.2017.10.017. Epub 2017 Oct 19.
- Praz F, Spargias K, Chrissoheris M, et al. Compassionate use of the PASCAL transcatheter mitral valve repair system for patients with severe mitral regurgitation: a multicentre, prospective, observational, first-in-man study. Lancet. 2017;390:773-780
- Kourkoveli P, Spargias K, Hahalis G. TAVR in 2017-What we know? What to expect? J Geriatr Cardiol. 2018 Jan;15(1):55-60. doi: 10.11909/j.issn.1671-5411.2018.01.005.
- FORWARD Study Investigators (Spargias K) Clinical Outcomes With a Repositionable Self-Expanding Transcatheter Aortic Valve Prosthesis: The International FORWARD Study. J Am Coll Cardiol. 2017;70(7):845-853.
- K Spargias, A Manginas, G Pavlides, M Khoury, G Stavridis, P Rellia, A Smirli, A Thanopoulos, M Balanika, S Polymeros, S Thomopoulou, G Athanassopoulos, G Karatasakis, R Mastorakou, S Lacoumenta, A Michalis, P Alivizatos, D Cokkinos. Transcatheter Aortic Valve Implantation: First Greek Experience. Hellenic J Cardiol 2008;49:397-407.
- Spargias K, Chrissoheris M, Halapas A, Nikolaou J, Tsolakis A, Bouboulis N, Pattakos S. Percutaneous Mitral Valve Repair Using the Edge-to-Edge Technique: First Greek Experience. Hellenic J Cardiol 2012;53:343-51.
- K Spargias, K Toutouzas, M Chrissoheris, Synetos A, Halapas A, Paizis I, Latsios G, Stahogianis K, Papametzelopoulos S, Zanos S, Pavlides G, Zacharoulis A, Antoniades A, Stefanadis C. The ATHENS TAVI Registry of newer generation transfemoral aortic valves. 30 day outcomes. Hellenic J Cardiol 2013;54:18-24.
- Chrissoheris M, Ziakas A, Halapas A, Hadjimiltiades S, Styliadis I, Karvounis C, Nikolaou I, Spargias K. Acute Invasive Hemodynamic Effects of Transcatheter Aortic Valve Implantation. J Heart Valve Disease 2016, Mar;25(2):162-172.
- K Spargias, A Halapas, M Chrissoheris, N Bouboulis, S Skardoutsos, J Nikolaou, A Tsolakis, C Mourmouris, S Pattakos. Transaortic Aortic Valve Replacement Using The Edwards Sapien-XT Valve And The Medtronic Corevalve. Initial Experience. Hellenic J Cardiol 2014;55:288-93.
- M Chrissoheris, A Tsangaris, A Halapas, K Spargias. Transcatheter Aortic Valve Replacement in Patients with Prior Chest Irradiation and Severe Aortic Stenosis. J Radiol Oncology 2016, In press.
- Spargias K, Dvir D. TAVR In Failed Bioprostheses. The Valve-In-Valve Approach. Cardiac Interv Today 2013; 45-8.
- Chrissoheris M, Halapas A, Nikolaou I, Boumboulis N, Pattakos S, Spargias K. The Abbott Vascular MitraClip: Patient Selection and How to Obtain the Best Outcomes. Hellenic J Cardiol. 2015;56 Suppl A:31-8.
- Tzikas A, Chrissoheris M, Halapas A, Spargias K. PorticoTM Transcatheter Heart Valve. Hellenic J Cardiol. 2015;56 Suppl A:15-9.
- Halapas A, Chrissoheris M, Bouboulis N, Skardoutsos S, Nikolaou I, Pattakos S, Spargias K. The SAPIEN-XT and SAPIEN-3 Valves: How to Implant and Obtain the Best Outcomes. Hellenic J Cardiol. 2015;56 Suppl A:9-14.
- Spargias K. The era of transcatheter valve therapy. Where are we? Hellenic J Cardiol. 2015;56 Suppl A:1-3.
- Wendler O, Walther T, Schroefel H, Lange R, Treede H, Fusari M, Rubino P, Thomas M; SOURCE investigators (K Spargias). Transapical aortic valve implantation: mid-term outcome from the SOURCE registry. Eur J Cardiothorac Surg 2013;43:505-11.
- Spargias K, Tzifa A, Chrissoheris M, Boumpoulis N, Nikolaou I, Pattakos E. Transapical closure of mitral prosthetic paravalvular leak. Hellenic J Cardiol 2013;54:397-400.
- A Halapas, M Chrissoheris, I Nikolaou, N Bouboulis, K Spargias. Challenging Transfemoral Valve-in-Valve Implantation in a Degenerated Stentless Bioprosthetic Aortic Valve. J Invasive Cardiol 2015;56:347-50
- Papavasileiou LP, Halapas A, Chrisocheris M, Bellos K, Bouboulis N, Pattakos S, Spargias K, Apostolopoulos T. Sudden Death After ranscatheter Aortic Valve Implantation. Are Bradyarrhythmias Always The Cause? JAFIB. 2015;8:39-41.
- K Spargias, G Dangas. Percutaneous Treatment of Left Side Cardiac Valves. C Tamburino and GP Ussia, Ed. Springer-Verlag, 2010, Milan, Italy.
- Chrissoheris M, Spargias K. “Balloon Aortic Valvuloplasty” in Textbook: Cardiac Valvular Medicine. Rajamannan NM (Ed.). Springer-Verlag, 2013, London, UK.
- Spargias K. Percutaneous aortic valve implantation: present and future. Controlled clinical application before the results of randomized trials. Hellenic J Cardiol 2009;50:237-44.
- K Spargias, M Gyöngyösi, R Hemetsberger, A Posa, N Pavo, IJ Pavo, K Huber, Z Petrasi, O Petnehazy, R Pogge von Strandmann, SR Stock, D Glogar, G Maurer, NM Rajamannan. Valvuloplasty with a Paclitaxel-Eluting Balloon Prevents Restenosis in an Experimental Model of Aortic Stenosis. J Heart Valve Disease 2014 Jul;23(4):484-91.
- Spargias K, Polymeros S, Dimopoulos A, Manginas A, Pavlides G, Balanika M, Smirli A, Stavridis G, Dangas G, Cokkinos DV. The predictive value and evolution of N-Terminal Pro-B-Type Natriuretic Peptide levels following Transcutaneous Aortic Valve Implantation. J Interv Cardiol 2011;24:462-9.
- Balanika M, Smyrli A, Samanidis G, Spargias K, Stavridis G, Karavolias G, Khoury M, Voudris V, Lacoumenta S. Anesthetic Management of Patients Undergoing Transcatheter Aortic Valve Implantation. J Cardiothorac Vasc Anesth 2013 Dec 5. [Epub ahead of print]
- Spargias K, Alexopoulos E, Thomopoulou S, Dimopoulos A, Manginas A, Pavlides G, Voudris V, Karatassakis G, Athanassopoulos G, Cokkinos DV. Effect of balloon valvuloplasty in patients with severe aortic stenosis on levels of N-terminal pro-B-type natriuretic peptide. Am J Cardiol 2009;104:846-849.
- Dvir D, Assali A, Spargias K, Kornowski R. Percutaneous aortic valve implantation in patients with coronary artery disease: review of therapeutic strategies. J Invasive Cardiol 2009;21:E237-41.
- Chrissoheris M, Ferti A, Spargias K. Early prosthetic valve endocarditis complicating repeated attempts at CoreValve implantation. J Invasive Cardiol 2011;23:E291-2.
- Spargias K, Milewski K, Debinski M, Buszman PP, Cokkinos DV, Pogge R, Buszman P. Drug delivery at the aortic valve tissues of healthy domestic pigs with a paclitaxel eluting valvuloplasty balloon. J Interv Cardiol 2009;22:291-8.
- Wilczek K, Chodór P, Przybylski R, Krasoń M, Niklewski T, Nadziakiewicz P, Głowacki J, Kusa J, Goddyn D, Spargias K, Zembala M. First in Poland transcatheter, transfemoral aortic valve implantation in elderly symptomatic high-risk patient with aortic stenosis-novel Zabrze experience. Kardiol Pol 2009;67:219-23.
- Kolettis TN, Spargias K, Stavridis GT. Combined transapical aortic valve implantation with coronary artery bypass grafting in a young patient with porcelain aorta. Hellenic J Cardiol 2009;50:79-82.
- Trendafilova D , Jorgova J, Simeonov P. Acknowledgement to Dr K Spargias. First Transfemoral Aortic Valve Implantation In Bulgaria. The Internet Journal of Cardiology 2010;9:2.
The HYGEIA Hospital Transcatheter Heart Valve Department is one of the leading research centers globally for in use and new transcatheter devices for the treatment of cardiac valve repairs. Many Greek patients could benefit from the new technologies being clinically tested within the department. Contact the Transcatheter Heart Valve Department to find out all about the current protocols. The treatments may have already been approved or may be investigational. These studies are usually sponsored by manufacturers and are offered free of charge.
Some research protocols at the Transcatheter Heart Valve Department:
MiCLASP. PASCAL in mitral valve repair
TriCLASP. PASCAL in tricuspid valve repair
CardioValve. Transcatheter Mitral valve replacement with CardioValve
TriCares. Transcatheter Tricuspid valve replacement
RESHAPE HF2 (επιδιόρθωση ανεπάρκειας μιτροειδούς, MitraClip, Abbott)
RHEIA Study (TAVI vs SAVR in women)
Popular Study (antithrombotic treatment after TAVI)
Aortic Valve Stenosis
The aortic valve is one of the 4 valves of the heart and it ensures blood flow from the heart to the rest of the body. The normal aortic valve opening is 3-4 cm2. The stenosis of the valve is considered significant when the opening is reduced to less than 1 cm2.
The degenerative mechanism that causes aortic valve stenosis (narrowing) is dynamic and evolving. The main features of this process are gradual stiffening and thickening of the valve with calcium deposits that progressively limit leaflet mobility and eventually narrow the valve. Approximately 5% of the elderly population (>75 years) have developed at least moderate aortic valve stenosis.
When the condition becomes severe, it develops rapidly and the prognosis is poor, with high morbidity and hassles for the patients and their families.
The most common symptoms of aortic valve stenosis are shortness of breath, angina and dizziness, up to syncopal attacks. After the onset of symptoms, average patient survival drops drastically.
Treatment
Pharmaceutical treatment can relieve some of the symptoms temporarily, but it does not affect or treat the valve condition. The only treatment is valve replacement, which could only be performed with open surgery until a few years ago. However, many patients are already of an advanced age when the symptoms appear and often suffer from other conditions that greatly increase the risk of conventional cardiac surgery, or even prohibit it. Therefore, surgical replacement is considered too risky in these patients and they often do not undergo surgery (it is estimated that 1 in every 2 patients who must undergo conventional surgical replacement of aortic valve is rejected). Prognosis in these patients is poor and morbidity long and painful.
Transcatheter Aortic Valve Replacement (Implantation): TAVR or TAVI is being successfully performed since 2007, initially on patients who could not have been treated in the past. Since 2011, TAVR has also been approved and is successfully performed on patients who could have undergone surgical replacement, but were considered high risk. With low single-digit mortality rates and fast recovery and return to daily activities, the benefits of this method are obvious.
In 2016, the results of the first clinical trials showed better survival with TAVR compared to the surgical replacement, even in lower (moderate) risk patients, and the use of the method has since been extended to these patients as well. In 2018, newer clinical trials produced similar results for the TAVR method in low-risk patients (essentially for all patients now). As a result, the method was recently (2019) approved and indicated by the FDA for application on essentially all patients with aortic valve stenosis.


Image 1. Illustration of implanted transcatheter valves in the position of the aortic valve
The Studies
The Transcatheter Aortic Valve Replacement is the most researched valve replacement method, as well as one of the most researched treatments in Cardiology and Cardiac Surgery in general.
The results of the randomized PARTNER-B study were announced in 2010, when it was found that the transcatheter method for treating aortic valve stenosis is changing the natural history of the disease and improves survival. Older patients with coexisting conditions, who would generally be treated conservatively due to the forbiddingly high surgical risk, underwent randomized transfemoral valve implantation and showed remarkable improvement of symptoms, reduced need for hospitalization by 54% and reduced morbidity by 45% as early as the first year. Consequently, the method was recognized for this patient population, who would otherwise be doomed to severe morbidity and very poor prognosis. It is estimated that there are at least 1,000 such patients in Greece (per year) who could benefit from the transcatheter approach.
The results of the randomized PARTNER-A study were announced in March 2011. PARTNER-A is significant in that it randomized high-risk patients (Logistic Euroscore ≥ 15%), who, however, were not rejected for surgical treatment. 30-day morbidity for the transcatheter procedure (3.4%) was almost 50% lower compared to the corresponding surgical replacement (6.7%), proving that they are at least equivalent treatments. Survival in the first year was 2.6% higher for the transcatheter method. Especially with regard to the transfemoral implantation, 30-day survival was significantly higher compared to the surgical replacement.
The results of PARTNER-A provided the first comparative assessment of the transcatheter implantation compared to the surgical replacement of the aortic valve, demonstrating that even high-risk patients who can undergo surgery enjoy just as good results with transcatheter implantation, and with all the benefits of a less invasive method. This population is estimated at around 25% of patients who undergo surgical replacement to date (patients with a Logistic Euroscore ≥ 15%) (approximately 300 patients/year in Greece, on top of the ~1000 deemed inoperable).
The first randomized clinical study that proved the superiority of the transcatheter method over the conventional surgical replacement was announced in the spring of 2014 (CoreValve US Pivotal High Risk Study). In this study, 795 high-risk patients (Logistic Euroscore average of 18%) who were eligible for conventional surgery were randomized to transcatheter or surgical replacement. The morbidity of those who underwent the transfemoral method was significantly lower both at 1 month (3.3% for the transcatheter, 4.5% for the surgical) and at 1 year (14.2% for the transcatheter, 19.1% for the surgical).
The results of the latest generation SAPIEN 3 valve by Edwards Lifesciences on 1,655 high and medium surgical risk patients were announced in the beginning of 2016. This study showed the lowest 30-day morbidity rates to date, i.e. 1.6% for the transfemoral procedure in high-risk patients and 1.1% in medium-risk patients.
The results of the PARTNER 2 study, which randomized 2,032 medium-risk patients (STS score 4%-8%) to transcatheter or surgical aortic valve replacement, were also announced in the beginning of 2016. The patients who underwent transfemoral valve implantation had better early survival as opposed to those who underwent conventional surgical replacement. The results of these studies provided the indication and established the transcatheter replacement in medium-risk patients (around 25% of patients undergoing surgical replacement up until that time).
The results of the PARTNER 3 and the EVOLUT Low Risk Trials were both announced at the beginning of 2019. They included 2,468 low-risk patients (STS < 4%) and during the 2-year follow-up, they showed that not only is TAVR safe and effective in these patients as well, but the incidence of death or stroke was halved compared to the conventional surgical replacement (PARTNER 3 and EVOLUT Low Risk Trials combined: 2.6% vs. 5.2%). In practical terms this means that if 200 such patients undergo TAVR as opposed to conventional surgery, 5 more will return for the 2-year follow-up alive and without having suffered a stroke. These are concrete results and will unavoidably lead to the generalized application of the TAVR in patients with aortic valve stenosis.
As of 2007, there has been wide clinical acceptance of the method (initially in Europe, followed by the USA and Japan) and the number of procedures performed followed a geometric progression, with an 40%-80% rise annually. More than 700,000 implantations have been performed to date (09/2019) globally (3,500 in Greece). In many countries, transcatheter implantations have surpassed the surgical ones. In most western European countries, more than 100 (and up to 300) transcatheter implantations are performed per 1 million population.
With regard to the clinical results in everyday practice, the announcements from the most recent register recordings and the studies show that 30-day morbidity in high/medium-risk patients who undergo transfemoral transcatheter replacement is around 2%-4%, while it is 1% in low-risk patients. The recorded 30-day morbidities for transthoracic and transaortic implantations are around 3%-8%.
Transcatheter implantations of all valve types currently available (SAPIEN 3, Edwards Lifesciences; Evolut PRO, Medtronic; Portico, Abbott; ACURATE neo and LOTUS Edge, Boston Scientific) are performed at HYGEIA Hospital, using all possible implantation methods (transfemoral, transaortic, transthoracic, subclavian route etc.). The Department staff’s experience exceeds 1,400 procedures, while 30-day morbidity of transfemoral procedures has been < 2%.





Image 2: The SAPIEN 3 (Edwards Lifesciences), Evolut PRO (Medtronic), Portico (Abbott), ACURATE neo and LOTUS Edge (Boston Scientific) transcatheter valves (from top left to bottom right)
Transcatheter Heart Valve Resilience
There is plenty of detailed follow-up information on many patients who carry transcatheter valves in the last 10 years and there have been no concerns as to their long-term resilience and functionality. Besides, they are manufactured by the same companies that manufacture surgical valves and undergo the same rigorous bench testing. It is expected that they will continue to operate well for just as long (i.e. 12-16 years). However, even if they malfunction, this will happen gradually and it will be possible to replace them with new transcatheter valves that will be implanted in the same simple manner. This is already being done on patients with malfunction of older surgically placed bioprosthetic valves, however, this time these patients are treated with the transcatheter method rather than undergoing a new surgical procedure.
Aortic Valve Regurgitation
Although it is rarer than aortic valve stenosis, aortic valve regurgitation causes the same serious problems and treatment is once again replacement of the faulty valve. Provided it can be performed, transcatheter replacement is the best choice for high-risk patients.
The Procedure
Transfemoral procedure
The procedure is performed through the femoral artery, similarly to a coronary angiography. Strict antisepsis measures are in place in the HYGEIA Hospital Hybrid Operating Room, where the procedure is performed. General anesthesia and intubation are not required, but patients are placed under sedation, so that they remain calm and can cooperate. The procedure usually lasts less than 30 minutes, but patients remain in the operating room for about 90 minutes total (preparation upon arrival and departure). The entry point of the valve into the femoral artery is sealed externally, while there is no incision on the skin.
Patients remain on bed rest for the first night postoperatively, at the Intensive Care Unit or High Dependency Unit, and then they are fully mobile and remain hospitalized in a simple ward for another 1-3 days. In the vast majority of patients (> 95%), the femoral arteries are suitable for valve implantation. It is generally accepted that transfemoral implantations are preferable, as they are less invasive and carry a lower risk.
Transaortic and transapical (transthoracic) procedure
Following the advancement and improvement of transcatheter valves, transaortic and transapical implantations are rarely performed these days (less than 5% of patients), and only when the femoral arteries are deemed unsuitable for transfemoral implantation (due to small size, stenosis, etc.). The procedure is performed under general anesthesia and the valve is implanted either through the aorta (transaortic), through a small incision between the ribs at the top of the chest, or directly through the heart (transapical), through a small incision between the ribs on the left, under the nipple. The procedure usually lasts less than 1 hour, but patients remain in the operating room for about 2 hours total (preparation upon arrival and departure). The incision on the skin between the ribs is around 3-5 cm long and is sutured closed. Patients remain on bed rest in the Intensive Care Unit for the first day postoperatively and then they are hospitalized in a simple ward for another 2-3 days.
These patients also enjoy the key benefits of the method, avoiding a sternotomy and extracorporeal circulation. The SAPIEN 3 and ACURATE neo valves may be implanted transaortically and transapically, the Evolut R and Portico transaortically, and the JenaValve transapically.
Eligibility testing
A patient’s eligibility is assessed through a series of tests as an outpatient, and rarely is 1-day hospitalization required. The tests include coronary angiography to check the heart arteries (invasive or bloodless with CT), cardiac CT, CT angiography of the limbs to determine their suitability for valve insertion, echocardiography, blood tests and any other exams deemed necessary, depending on each patient. Patients are also evaluated by the entire medical team that will participate in the procedure (cardiologists, cardiac surgeons, anesthesiologists, vascular surgeons, etc., i.e. the Heart Team). After testing, they decide on: the implantation method (transfemoral, transaortic, transapical, etc.), most suitable valve type (for anatomical and other reasons, one type of valve is often preferable to another in each patient) and suitable valve size.
Through this procedure, it is also possible to manage any significant stenoses encountered in the coronary arteries using angioplasty and stents. Finally, palliative valvuloplasty (valve opening with balloon) may be performed on gravely ill patients, so they may undergo valve implantation at a later date.
Mitral Valve Regurgitation
It is the most frequent valve disease and it is estimated that 8%-10% of people aged over 75 may develop at least moderate mitral valve regurgitation. The inability of this valve to close tightly results in part of the blood, which was to be channeled to the entire body through the aorta, flowing back into the lungs.
Severe mitral valve regurgitation causes heart failure symptoms (fatigue and shortness of breath during natural fatigue, up to acute pulmonary edema) and predisposition for developing atrial fibrillation (arrhythmia) and stroke.
Mitral valve regurgitation is distinguished into functional (usually due to coronary disease or dilated cardiomyopathy) and organic (often degenerative, which causes leaflet prolapse). Pharmaceutical treatment may manage and temporarily improve some of the symptoms caused by severe mitral valve regurgitation. However, given that the problem is primarily mechanical, it cannot treat it.
Surgical treatment of the functional regurgitation of the mitral valve is attempted only when the patient also suffers from coronary disease and must undergo aortocoronary bypass surgery for precisely this reason. Otherwise the benefit is unconfirmed and the risk of the procedure high. This is also the reason why patients rarely undergo surgical procedure just to treat the valve regurgitation. Transcatheter repair is applicable to them.
Surgical treatment of organic valve regurgitation usually involves open or endoscopic surgical repair. However, at times, this is not possible, in which case, it is replaced by a prosthetic valve. Nevertheless, there are many such patients who do not eventually undergo surgical treatment because they are deemed high risk for various reasons. Transcatheter repair is applicable to them.
MitraClip: Transcatheter mitral valve regurgitation repair system
The MitraClip system (Abbott Vascular) is based on the transcatheter placement of one or more clips in the mitral valve and aims to reduce or even eliminate the regurgitation. This minimally invasive mitral valve repair mimics the open surgery method by creating a double orifice (Alfieri method) and increases the treatment choices available to patients who suffer from severe valve regurgitation.
The MitraClip device places a clip in the mitral valve, without opening the patient’s chest and without incisions. The guide catheter is introduced through the femoral vein to reach the heart, similarly to a coronary angiography. The procedure is performed under general anesthesia, without using a heart-lung machine (extracorporeal circulation) and without a sternotomy. Hospitalization is two to three days postoperatively.
It has been proven to reduce the symptoms of heart failure and improve the quality of life in select patients with mitral valve regurgitation, similarly to the surgical method, but much safer. There is strong evidence available currently suggesting that it increases survival compared to conservative pharmaceutical treatment.
The system consists of three main subsystems:
- A steerable guide catheter
- A clip delivery system
- A MitraClip device (implant)

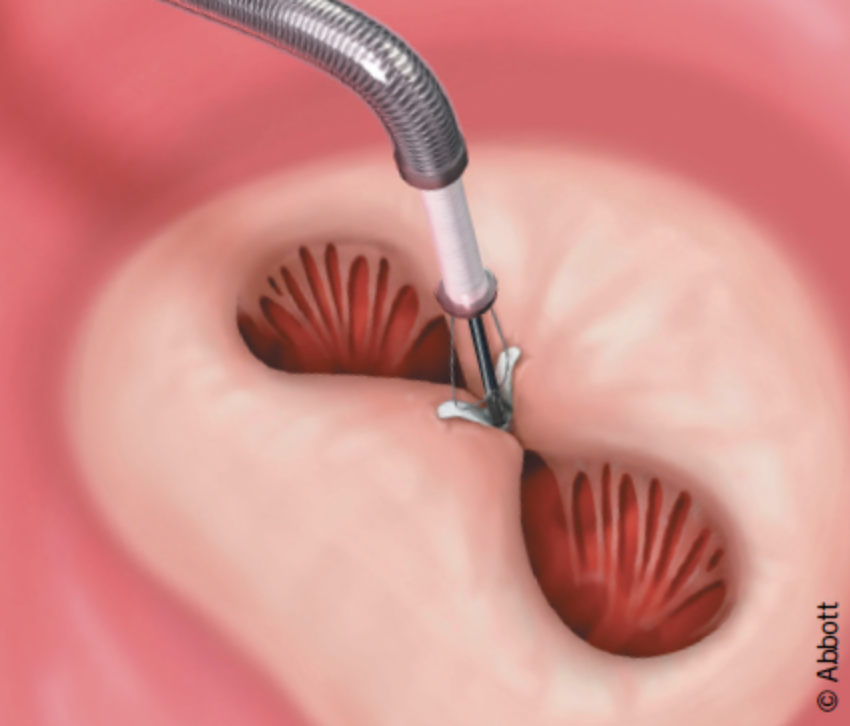
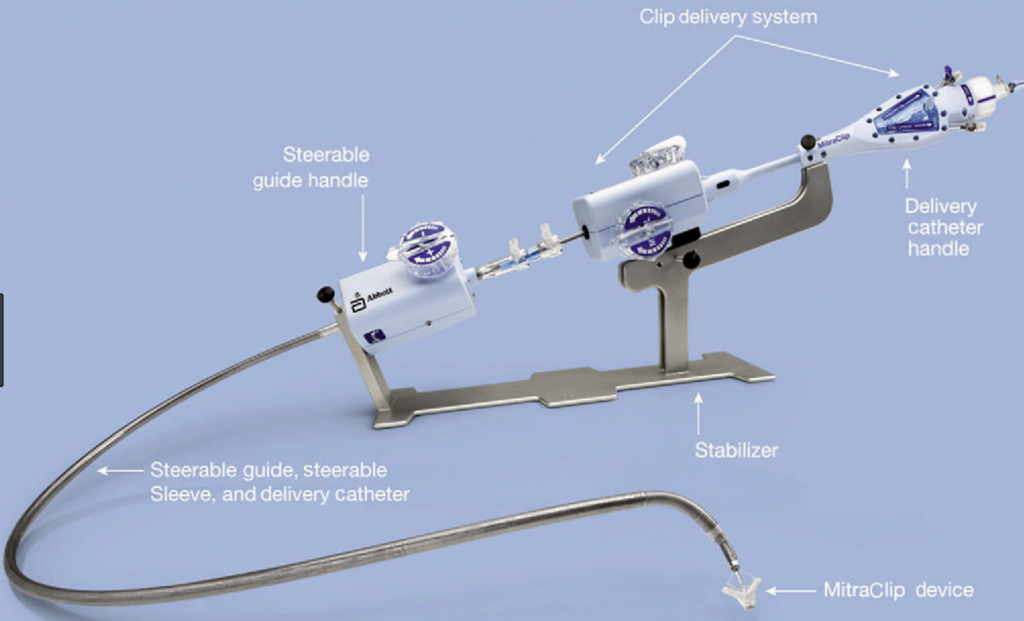
Image 1. The MitraClip system
The MitraClip is the first transcatheter mitral valve regurgitation repair system to receive the European CE certification in 2008 and be approved by the FDA in 2013, while more than 80,000 procedures have been performed globally to date.
The COAPT clinical trial, announced in 2018, included 603 patients with functional mitral valve regurgitation and compared the MitraClip with the conservative conventional treatment. It is extremely important that treatment using the MitraClip method showed significant and major reduction in morbidity, resulting in the US FDA extending the treatment indications in 2019.
In Greece, these procedures were performed for the first time by the HYGEIA Hospital Transcatheter Heart Valve Department in October 2011 and were met with great success. The program has been successfully continuing since then and more than 250 clips have been implanted.
The clinical application of the transcatheter mitral valve regurgitation repair using the PASCAL system by Edwards Lifesciences was approved in February 2019.
The HYGEIA Hospital Transcatheter Heart Valve Department was among the main investigators of the CLASP trial, which led to the PASCAL system receiving the CE mark due to its excellent results. The PASCAL system is currently used for the transcatheter repair of the mitral valve. The technique is similar to the one used for the MitraClip system, which is being successfully applied at HYGEIA for many years. The first commercial application in Greece was at the HYGEIA Hospital Transcatheter Heart Valve Department in 2019.
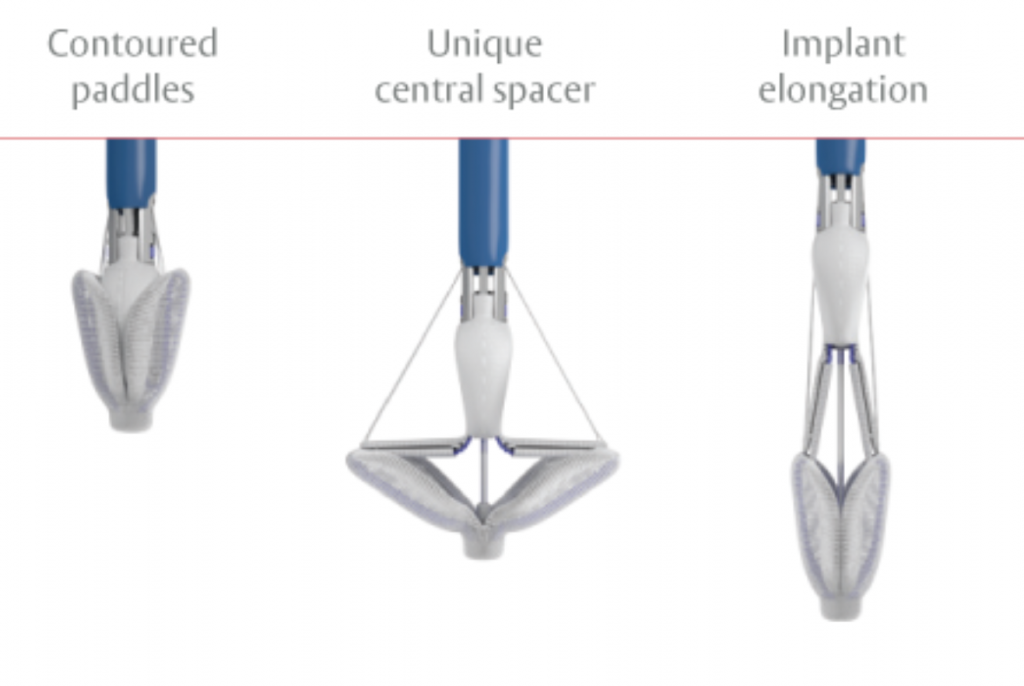
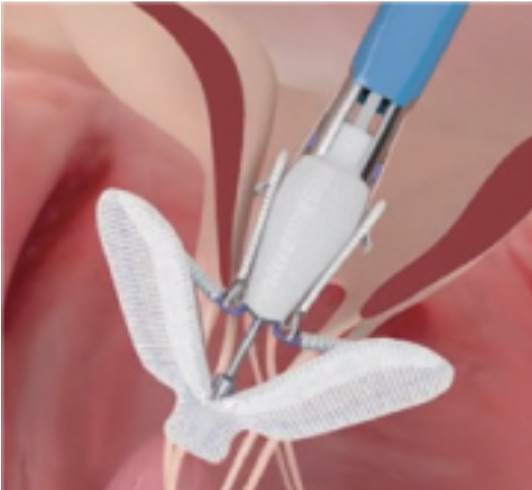
Image 2. The PASCAL system
Possible treatment indications for MitraClip/PASCAL
The implantation of the clip may significantly reduce or even eliminate mitral valve regurgitation in the following cases:
- Significant functional mitral valve regurgitation that does not require surgical treatment of coexisting coronary artery disease.
- Significant organic mitral valve regurgitation in high surgical risk patients.
The anatomical eligibility criteria are examined using transthoracic and intraesophageal echocardiography (Doppler ultrasound).
- © 2007-2024 HYGEIA S.M.S.A.
- Personal Data Protection Policy
- COOKIES Policy
- Terms of Use
- Privacy Policy
- Credits
- Sitemap
- Made by minoanDesign
Ο ιστότοπoς μας χρησιμοποιεί cookies για να καταστήσει την περιήγηση όσο το δυνατόν πιο λειτουργική και για να συγκεντρώνει στατιστικά στοιχεία σχετικά με τη χρήση της. Αν θέλετε να λάβετε περισσότερες πληροφορίες πατήστε Περισσότερα ή για να αρνηθείτε να παράσχετε τη συγκατάθεσή σας για τα cookies, πατήστε Άρνηση. Συνεχίζοντας την περιήγηση σε αυτόν τον ιστότοπο, αποδέχεστε τα cookies μας.
Αποδοχή όλων Άρνηση όλων ΡυθμίσειςCookies ManagerΡυθμίσεις Cookies
Ο ιστότοπoς μας χρησιμοποιεί cookies για να καταστήσει την περιήγηση όσο το δυνατόν πιο λειτουργική και για να συγκεντρώνει στατιστικά στοιχεία σχετικά με τη χρήση της. Αν θέλετε να λάβετε περισσότερες πληροφορίες πατήστε Περισσότερα ή για να αρνηθείτε να παράσχετε τη συγκατάθεσή σας για τα cookies, πατήστε Άρνηση. Συνεχίζοντας την περιήγηση σε αυτόν τον ιστότοπο, αποδέχεστε τα cookies μας.
























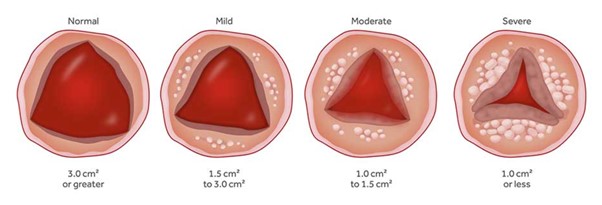

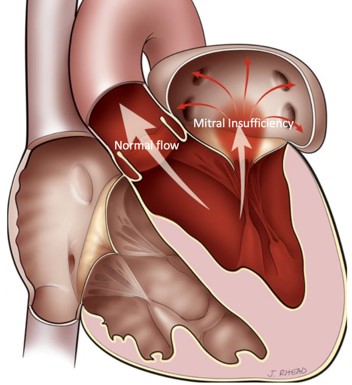
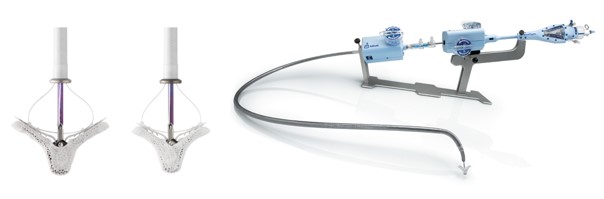

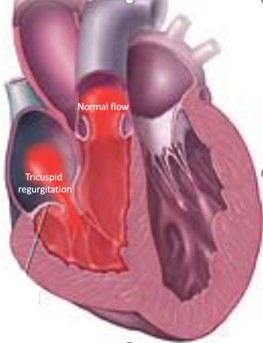
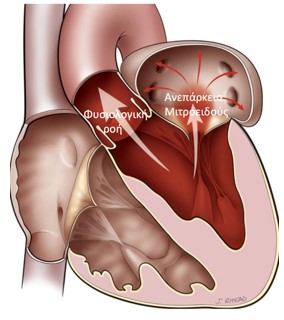
 SAPIEN XT valve implantation
SAPIEN XT valve implantation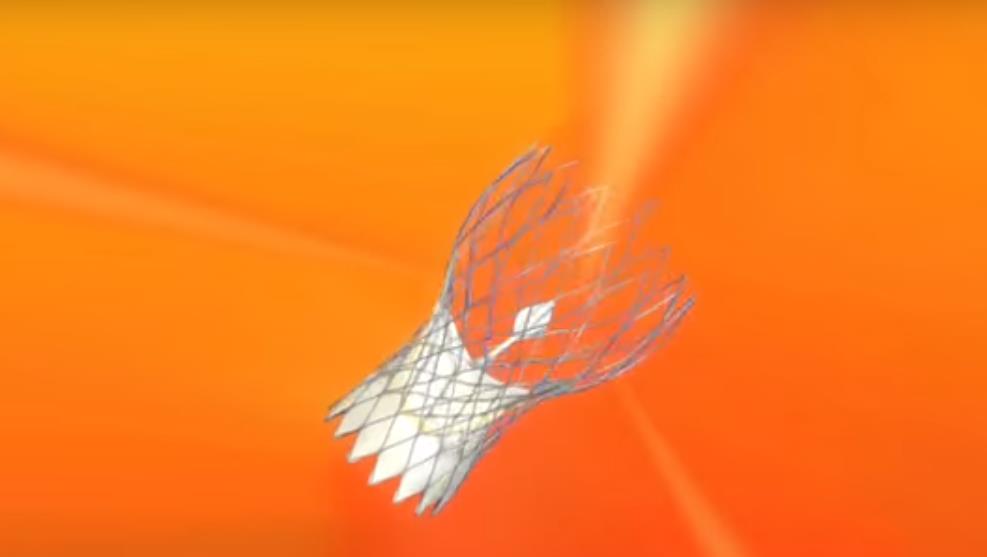 CoreValve implantation
CoreValve implantation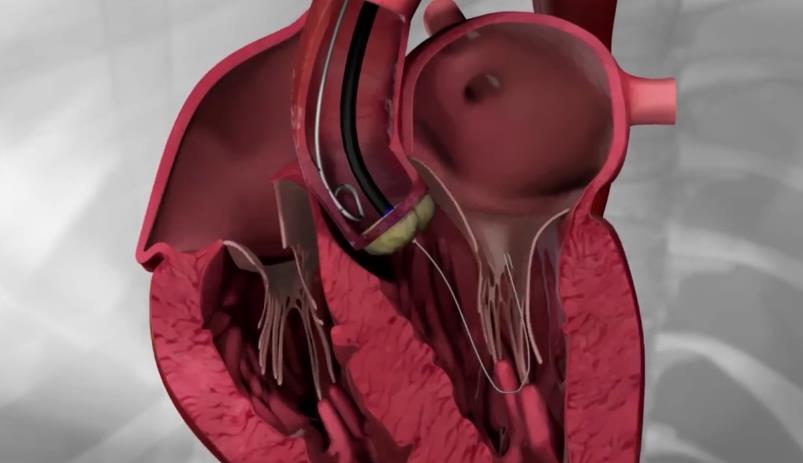 Portico valve implantation
Portico valve implantation JENA valve implantation
JENA valve implantation Mitraclip surgery in 3D
Mitraclip surgery in 3D Inverview at Ant1 channel, 2012
Inverview at Ant1 channel, 2012 Developments - Trancatheter Heart Valve Therapy, Interview at Iatronet
Developments - Trancatheter Heart Valve Therapy, Interview at Iatronet














